��Ŀ����
�����1����⣺__________________������⡣
���أ�__________________��װ�ã�Ҳ����__________________��װ�ý������ػ���ۡ�
��2�����ɵ��ص�����
��_________��
��__________________��
��__________________��
��3�����ص�����
������_________�������������Ϸ���_________��Ӧ��
������_________�������������Ϸ���_________��Ӧ��
��4���������Һ�����ʵ��
�������Һ����Ĺ��̣�����_________���̣�������������������������ԭ��Ӧ�Ĺ��̡�
��5�����ӵķŵ�
����������������ʧ���ӵĹ��̳Ʒŵ硣���������ӻ���������ӷֱ���������������ʱ�����ȷŵ����_____________�����ӡ�
д�����������������������ŵ�ĵ缫����ʽ��
Ag+��Fe3+��H+��Zn2+��OH-��Br-��S2-��![]()
____________________________________��
____________________________________��
��1��ʹ����ͨ���������Һ��������������������������ԭ��Ӧ�Ĺ��� �����ڵ�������������ԭ��Ӧ �ѵ���ת��Ϊ��ѧ��
��2���������缫
��ֱ����Դ
�۵������Һ������̬�����
��3����Դ���� ���� ��Դ���� ��ԭ
��4���������Һ�ĵ��
��5��������ǿ�������ӻ�ԭ��ǿ����
�����ӣ�Ag++e-![]() Ag Fe3++e-
Ag Fe3++e-![]() Fe2+ 2H++2e-
Fe2+ 2H++2e-![]() H2�� Zn2++2e-
H2�� Zn2++2e-![]() Zn
Zn
�����ӣ�4OH--4e-![]() 2H2O+O2�� 2Br--2e-
2H2O+O2�� 2Br--2e-![]() Br2 S2--2e-
Br2 S2--2e-![]() S
S ![]() +H2O-2e-
+H2O-2e-![]()
![]() +2H+
+2H+

 �߽�������ϵ�д�
�߽�������ϵ�д�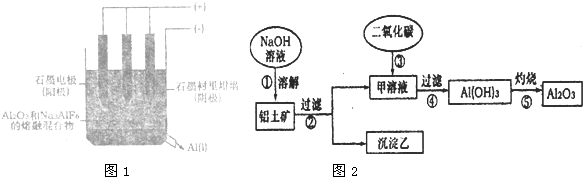
 ��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�
��Ҫ�����ͼ���������б�Ҫ�����Ӳ���գ�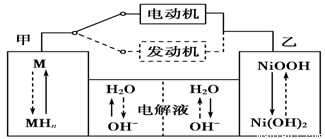
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����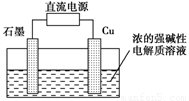
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��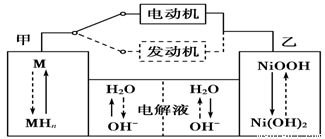
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��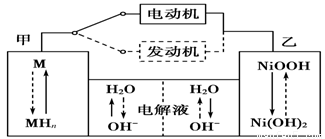
 2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����
2Ni(OH)2������������Ϣ�жϣ���϶��������»����ʱ����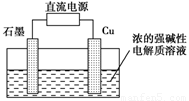
 Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��
Cu2O��H2���������ĵ缫��Ӧʽ��_____________________��