��Ŀ����
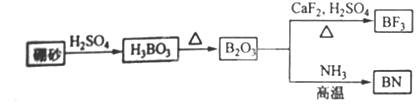
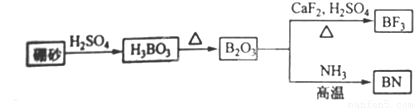
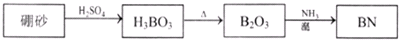
������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BN������ͼ��ʾ��

����H3BO3�Ʊ�B2O3�Ļ�ѧ����ʽ��_________��
��ijʵ��С������������Ӧԭ��(B2O3 + 2NH3 2BN + 3H2O)������װ���Ʊ�������
2BN + 3H2O)������װ���Ʊ�������
��ijʵ��С������������Ӧԭ��(B2O3 + 2NH3
 2BN + 3H2O)������װ���Ʊ�������
2BN + 3H2O)������װ���Ʊ�������
��֪��BN�������ܱ�����������������ˮ��B2O3��������ˮ����������ˮ��
��1�����Ӻ�װ�ú��ڸ����Ӵ�ͿĨ����ˮ��Ȼ��ͨ��N2��������ԡ�֤�����������õ�������_____________��ͨ��N2����һ������______________��
��2��ƿB��װ��������Һ��������_____________��
��3����Ӧ����ʱ���رյ�¯�ͻ���K���������õ��ɼмн�A��B֮�����Ƥ�ܣ�ֱ��___________��ת�Ʋ����û�мн���Ƥ�ܿ��ܹ۲쵽��������__________��
��4������ȥ������δ��Ӧ��B2O3���õ�������BN���壬ʵ�����������__________��
��1�����Ӻ�װ�ú��ڸ����Ӵ�ͿĨ����ˮ��Ȼ��ͨ��N2��������ԡ�֤�����������õ�������_____________��ͨ��N2����һ������______________��
��2��ƿB��װ��������Һ��������_____________��
��3����Ӧ����ʱ���رյ�¯�ͻ���K���������õ��ɼмн�A��B֮�����Ƥ�ܣ�ֱ��___________��ת�Ʋ����û�мн���Ƥ�ܿ��ܹ۲쵽��������__________��
��4������ȥ������δ��Ӧ��B2O3���õ�������BN���壬ʵ�����������__________��
��2H3BO3  B2O3+ 3H2O
B2O3+ 3H2O
��1�������Ӵ�������ĭ������ ��ȥװ���е�����
��2������δ��Ӧ�İ���
��3��װ����ȴ������ �� ƿB�е�ϡ���ᵹ����ƿA�У������������𰸣�
��4�����ֲ�Ʒ������������ˮ����ֽ������ȹ��ˣ�����ˮϴ������2��3�Σ����
 B2O3+ 3H2O
B2O3+ 3H2O��1�������Ӵ�������ĭ������ ��ȥװ���е�����
��2������δ��Ӧ�İ���
��3��װ����ȴ������ �� ƿB�е�ϡ���ᵹ����ƿA�У������������𰸣�
��4�����ֲ�Ʒ������������ˮ����ֽ������ȹ��ˣ�����ˮϴ������2��3�Σ����

��ϰ��ϵ�д�
�����Ŀ

 ����B3N3H6�Ķ��ȴ�����
����B3N3H6�Ķ��ȴ�����