��Ŀ����
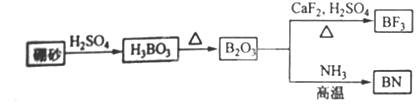
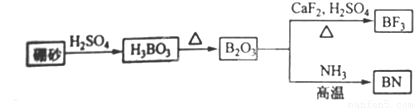
������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN����ͼ��ʾ��

��ش��������⣺
��1����B2O3�Ʊ�BN�Ļ�ѧ����ʽ��
��2����̬Bԭ�ӵĵ����Ų�ʽΪ
��3����BF3�����У�F-B-F�ļ�����
��4��BF3����NaF���ÿ�����NaBF4��
�����幹��Ϊ
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ

��ش��������⣺
��1����B2O3�Ʊ�BN�Ļ�ѧ����ʽ��
B2O3+2NH3
2BN+3H2O
| ||
B2O3+2NH3
2BN+3H2O
��
| ||
��2����̬Bԭ�ӵĵ����Ų�ʽΪ
1s22s22p1
1s22s22p1
��B��N��ȣ��縺�Խϴ����N
N
��BN��BԪ�صĻ��ϼ�Ϊ+3��
+3��
����3����BF3�����У�F-B-F�ļ�����
120��
120��
��Bԭ�ӵ��ӻ��������Ϊsp2�ӻ�
sp2�ӻ�
����4��BF3����NaF���ÿ�����NaBF4��
| BF | - 4 |
��������
��������
����5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ
���ۼ�
���ۼ�
�����������Ϊ���»���
���»���
����������1���ɹ������̿�֪��B2O3��NH3��Ӧ����BN������ԭ���غ��֪������ˮ���ɣ�
��2����ԭ�Ӻ��������ĿΪ5�����ݺ�������Ų�������д��ͬ���ڴ����ҵ縺��������ǿ��B��IIIA��Ԫ�أ�Ϊ+3�ۣ�
��3���۲���ӶԻ���������Ϊ�����ӵ����幹���ǡ��۲���Ӷԡ���ų�Ľ�������жϼ۲���Ӷ�������ȷ�����ͺ��ӻ���ʽ��
��4���۲���ӶԻ��������ж���ҵ��ӶԺµ��Ӷ���Ŀ��
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���ۼ������������Ϊ���»�����
��2����ԭ�Ӻ��������ĿΪ5�����ݺ�������Ų�������д��ͬ���ڴ����ҵ縺��������ǿ��B��IIIA��Ԫ�أ�Ϊ+3�ۣ�
��3���۲���ӶԻ���������Ϊ�����ӵ����幹���ǡ��۲���Ӷԡ���ų�Ľ�������жϼ۲���Ӷ�������ȷ�����ͺ��ӻ���ʽ��
��4���۲���ӶԻ��������ж���ҵ��ӶԺµ��Ӷ���Ŀ��
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���ۼ������������Ϊ���»�����
����⣨1���ɹ������̿�֪��B2O3��NH3��Ӧ����BN������ԭ���غ��֪������ˮ���ɣ���Ӧ����ʽΪ��B2O3+2NH3
2BN+3H2O��
�ʴ�Ϊ��B2O3+2NH3
2BN+3H2O��
��2����ԭ�Ӻ��������ĿΪ5��ԭ�ӵĵ����Ų�ʽΪ1s22s22p1��ͬ���ڴ����ҵ縺��������ǿ�����Ե縺��N��B��B�ڢ�A��Ԫ�أ�Ϊ+3�ۣ�
�ʴ�Ϊ��1s22s22p1��N��3��
��3��BF3���ӵ�����ԭ��Bԭ���Ϻ���3���� ��������ԭ���ϵŵ��Ӷ���=
��a-xb��=
��0-3��1��=0������BF3���ӵ�VSEPRģ����ƽ�������ͣ�����ԭ����û�й¶Ե��ӣ�������ռ乹�;���ƽ�������Σ�������120�㣬BF3���ӵ�����ԭ��Bԭ�ӵļ۲���Ӷ���Ϊ��3������sp2�ӻ���
�ʴ�Ϊ��120�㣻sp2�ӻ���
��4��BF3����NaF���ÿ�����NaBF4��BF4-��Bԭ�ӵļ۲���Ӷ�=4+
=4���������в����µ��Ӷԣ�Ϊ��������ṹ���ʴ�Ϊ���������壻
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���ۼ������������Ϊ���»������ʴ�Ϊ�����ۼ������»�����
| ||
�ʴ�Ϊ��B2O3+2NH3
| ||
��2����ԭ�Ӻ��������ĿΪ5��ԭ�ӵĵ����Ų�ʽΪ1s22s22p1��ͬ���ڴ����ҵ縺��������ǿ�����Ե縺��N��B��B�ڢ�A��Ԫ�أ�Ϊ+3�ۣ�
�ʴ�Ϊ��1s22s22p1��N��3��
��3��BF3���ӵ�����ԭ��Bԭ���Ϻ���3���� ��������ԭ���ϵŵ��Ӷ���=
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��120�㣻sp2�ӻ���
��4��BF3����NaF���ÿ�����NaBF4��BF4-��Bԭ�ӵļ۲���Ӷ�=4+
| 1 |
| 2 |
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���ۼ������������Ϊ���»������ʴ�Ϊ�����ۼ������»�����
���������⿼��ṹ����λ�ù�ϵ����������Ų����ɡ��ӻ�������縺�Եȣ���Ŀ�ۺ��Խϴ��ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ����B3N3H6�Ķ��ȴ�����
����B3N3H6�Ķ��ȴ�����