��Ŀ����
13����һ�������NaOH��Һ�ֳ����ȷݣ�һ����pH=2��һԪ��HA��Һ�кͣ���������Һ�����ΪV1����һ����pH=2��һԪ��HB��Һ�кͣ���������Һ�����ΪV2��������������ȷ���ǣ�������| A�� | ��V1��V2����˵��HA�����Ա�HB������ǿ | |
| B�� | ��V1��V2����˵��HA�����Ա�HB�������� | |
| C�� | ��Ϊ��������Һ��pH��ȣ���V1һ������V2 | |
| D�� | HA��HB�ֱ��NaOH�кͺ����õ���Һ��һ�������� |
���� pH ��Ϊ2��һԪ��HA��HB��������Һ��H+����Ũ����ȣ���Խ�������Ũ��Խ�����������Ʒ�Ӧʱ���ĵ����ԽС���������ĵ����������Խ�ǿ����V1��V2����˵�� HA �����Ա�HB������ǿ����V1=V2����˵�� HA ��������HB��������ͬ��
��� �⣺pH ��Ϊ2��һԪ��HA��HB��������Һ��H+����Ũ����ȣ���Խ�������Ũ��Խ�����������Ʒ�Ӧʱ���ĵ����ԽС���������ĵ����������Խ�ǿ����V1��V2����˵�� HA �����Ա�HB������ǿ����V1=V2����˵�� HA ��������HB��������ͬ��
A�������Ϸ�����֪��Vl��V2����˵�� HA �����Ա�HB������ǿ����A��ȷ��
B�������Ϸ�����֪��Vl��V2����˵�� HA �����Ա�HB������ǿ����B����
C����Ϊ��֪�������������ǿ����ϵ�����Բ����ж�V1��V2����Դ�С����C����
D����HA��HBΪ���ᣬ��HA��HB�ֱ��NaOH�кͺ����õ���Һ�Լ��ԣ���D����
��ѡA��
���� ���⿼������ǿ���Ķ����жϣ���Ŀ�Ѷ��еȣ�ע�������������Һ��H+����Ũ�����ʱ����Խ�������Ũ��Խ����һ�ص�����жϣ�

��ϰ��ϵ�д�
�����Ŀ
3����֪Ԫ�ص�ij�����ʡ�X����ԭ�Ӱ뾶�������ԡ��ǽ����Ե�һ����Ҳ��Ԫ�ص�һ�ֻ������ʣ��������13��Ԫ�ص�X����ֵ��
�Խ��Ԫ��������֪ʶ����������⣺
��1��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�ص�X��ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlCl3�еĻ�ѧ�������ǹ��ۼ���
��2�������ϱ����������ݣ���������Ԫ�ص�X����ֵ��С��Ԫ�صĽ����Ի�ǽ�����ǿ��֮��Ĺ�ϵԪ��X����ֵԽ��Ԫ�صķǽ�����Խǿ����Ԫ��X����ֵԽС��Ԫ�صĽ�����Խǿ���������ڶ�����Ԫ�أ������������⣩��X����ֵ��С��ԭ�Ӱ뾶֮��Ĺ�ϵԭ�Ӱ뾶ԽС��X����ֵԽ��
��3������Ԥ��Br��IԪ�ص�X��ֵ�Ĵ�С��ϵBr����I��
��4��ij����������к���S-N��������Ϊ�ù������Ӷ�ƫ����Nԭ�ӣ���Ԫ�ط��ţ���
| Ԫ�� | Al | B | Be | C | Cl | F | Li |
| X����ֵ | 1.5 | 2.0 | 1.5 | 2.5 | 2.8 | 4.0 | 1.0 |
| Ԫ�� | Mg | Na | O | P | S | Si | |
| X����ֵ | 1.2 | 0.9 | 3.5 | 2.1 | 2.5 | 1.7 |
��1��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�ص�X��ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlCl3�еĻ�ѧ�������ǹ��ۼ���
��2�������ϱ����������ݣ���������Ԫ�ص�X����ֵ��С��Ԫ�صĽ����Ի�ǽ�����ǿ��֮��Ĺ�ϵԪ��X����ֵԽ��Ԫ�صķǽ�����Խǿ����Ԫ��X����ֵԽС��Ԫ�صĽ�����Խǿ���������ڶ�����Ԫ�أ������������⣩��X����ֵ��С��ԭ�Ӱ뾶֮��Ĺ�ϵԭ�Ӱ뾶ԽС��X����ֵԽ��
��3������Ԥ��Br��IԪ�ص�X��ֵ�Ĵ�С��ϵBr����I��
��4��ij����������к���S-N��������Ϊ�ù������Ӷ�ƫ����Nԭ�ӣ���Ԫ�ط��ţ���
4����һ�������£���A��B��C����Ȳ������ɵĻ������4g���ڴ�������������H2�����ӳɷ�Ӧ������4.4g���ֶ�Ӧ��������������������һ���У�������
| A�� | ���� | B�� | ���� | C�� | ���� | D�� | ���� |
1����Na2O2���뵽����Mg2+��NH4+�Ļ��Һ�в��ȣ�������������������ʵ��������Na2O2�����ʵ����Ĺ�ϵ����ͼ��ʾ����ԭ���Һ��Al3+��Mg2+��NH4+�����ʵ����ֱ��ǣ�������
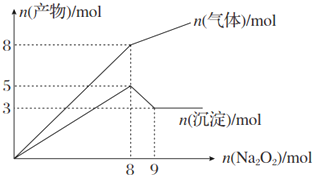

| A�� | 2 mol��3 mol��4 mol | B�� | 2 mol��3 mol��8 mol | ||
| C�� | 3 mol��2 mol��8 mol | D�� | 3 mol��2 mol��4 mol |
8�������������������еķ�ӦҺ�����γ���Ȫ���ǣ�������
| A�� | HCl ��ˮ�� | B�� | NH3��ˮ�� | ||
| C�� | SO2��Ũ����������Һ�� | D�� | Cl2������ʳ��ˮ�� |
2����7.4g Na2CO3•10H2O �� NaHCO3��ɵĻ��������ˮ���100mL��Һ������c��Na+��=0.6mol/L�����ѵ������Ļ������������أ�������������ǣ�������
| A�� | 3.18g | B�� | 2.12g | C�� | 4.22g | D�� | 5.28g |
 ��
�� ��
��