��Ŀ����
��ҵ�Ϻϳɰ���һ�������½������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H=-92.44kJ/mol���䲿�ֹ���������ͼ1��ʾ��

��1���ϳɰ�����Ҫ��ԭ�����У�����ȡ��______��������Դ��______��
��2����ԭ�������о���������Ŀ����______��
��3���豸A��������______���豸B��������______��
��4����20��50Mpaʱ����ҵ�ϳɰ�ѡ����400-500����¶Ƚ��з�Ӧ����Ҫԭ����______��
��5���ݡ���ѧ����־������ϣ����ѧ���ڳ�ѹ�½�������������ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ��أ���ͼ2���У���͵��ڵ缫�Ϻϳ��˰�����ת���ʴﵽ��78%����������ӦΪ______��������ӦΪ______��

��1���ϳɰ�����Ҫ��ԭ�����У�����ȡ��______��������Դ��______��
��2����ԭ�������о���������Ŀ����______��
��3���豸A��������______���豸B��������______��
��4����20��50Mpaʱ����ҵ�ϳɰ�ѡ����400-500����¶Ƚ��з�Ӧ����Ҫԭ����______��
��5���ݡ���ѧ����־������ϣ����ѧ���ڳ�ѹ�½�������������ϡ�͵ĵ����ֱ�ͨ��һ�����ȵ�570��ĵ��أ���ͼ2���У���͵��ڵ缫�Ϻϳ��˰�����ת���ʴﵽ��78%����������ӦΪ______��������ӦΪ______��
��1�����ݹ�ҵ�����У��������Կ�������������ˮ��̼�⻯����ʴ�Ϊ��������ˮ��̼�⻯���
��2����ҵ���ںϳɰ��Ĺ����У������ԭ�Ͻ��о�������ֹ��������ý�ж�������С�����ԣ��ʴ�Ϊ����ֹ�����ж���
��3���ϳɰ����豸�����У�ѹ�������ϳ�����������������������ѭ��ѹ�������ʴ�Ϊ����������ѭ��ѹ������
��4���ϳɰ��Ĺ����У�Ϊ���Ϸ�Ӧ���ʺ��Ƚϴ����Ļ��Դ�Ҫѡ�����˵��¶�400-500�棬�ʴ�Ϊ�����¶��´����Ļ��Դ�
��5����͵��ڵ缫�Ϻϳ��˰��ĵ����У���������������ʧ���ӵ�������Ӧ����H2-2e-�TH2���������ǵ��������õ��ӵĻ�ԭ��Ӧ����N2+6e-+6H+�T2NH3���ʴ�Ϊ��H2-2e-�T2H+��N2+6e-+6H+�T2NH3��
��2����ҵ���ںϳɰ��Ĺ����У������ԭ�Ͻ��о�������ֹ��������ý�ж�������С�����ԣ��ʴ�Ϊ����ֹ�����ж���
��3���ϳɰ����豸�����У�ѹ�������ϳ�����������������������ѭ��ѹ�������ʴ�Ϊ����������ѭ��ѹ������
��4���ϳɰ��Ĺ����У�Ϊ���Ϸ�Ӧ���ʺ��Ƚϴ����Ļ��Դ�Ҫѡ�����˵��¶�400-500�棬�ʴ�Ϊ�����¶��´����Ļ��Դ�
��5����͵��ڵ缫�Ϻϳ��˰��ĵ����У���������������ʧ���ӵ�������Ӧ����H2-2e-�TH2���������ǵ��������õ��ӵĻ�ԭ��Ӧ����N2+6e-+6H+�T2NH3���ʴ�Ϊ��H2-2e-�T2H+��N2+6e-+6H+�T2NH3��

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ
�ϳɰ��Ի�ѧ��ҵ������ҵ������Ҫ���壮
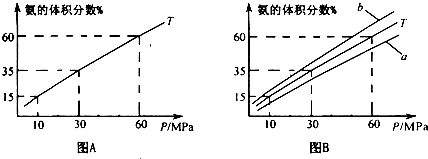
��1����ϳ����а�1��4�����ʵ���֮�ȳ���N2��H2���а��ĺϳɣ�ͼAΪTCʱƽ�������а��������������ѹǿ��P���Ĺ�ϵͼ��

��д����ҵ�Ϻϳɰ��Ļ�ѧ����ʽ ��
��ͼA�а������������Ϊ15.00%ʱ��H2��ת����= ��
��ͼB��T=500°C���¶�Ϊ450C��Ӧ�������� ��ѡ����ĸ��a����b������ѡ���������
����ͼ��֪������ѹǿ�����ԭ�ϵ������ʣ�������ʵ�ʿ�������ѹǿ������������ ��д��һ�����ɣ���
��2���ϳɰ�������������ɼ�����ˮ��Ӧ�Ƶã���Ӧ���Ȼ�ѧ����ʽΪ��
CH4��g��+H2O CO��g��+3H2��g������H=+QkJ/mol��Q��0��
CO��g��+3H2��g������H=+QkJ/mol��Q��0��
��3��һ���¶��£���2L�����з���������Ӧ�������ʵ����ʵ����仯���±�
�ٷ����������ݣ��ж�5?7min֮�䷴Ӧ�Ƿ���ƽ��״̬ ����ǡ�����
ǰ5minƽ����Ӧ����v��CH4��= ��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������ٵ�ԭ������� ������ĸ����
a?����CH4 b?�����¶�c?����ѹǿd?����H2
���������¶Ȳ��䣬��1L��������ʼ����0.15mol CH4.0.45mol H2O�� mol CO�� mol H2���ﵽƽ��ʱCH4������ٷֺ������һ��Ͷ����ͬ��
��1����ϳ����а�1��4�����ʵ���֮�ȳ���N2��H2���а��ĺϳɣ�ͼAΪTCʱƽ�������а��������������ѹǿ��P���Ĺ�ϵͼ��

��д����ҵ�Ϻϳɰ��Ļ�ѧ����ʽ ��
��ͼA�а������������Ϊ15.00%ʱ��H2��ת����= ��
��ͼB��T=500°C���¶�Ϊ450C��Ӧ�������� ��ѡ����ĸ��a����b������ѡ���������
����ͼ��֪������ѹǿ�����ԭ�ϵ������ʣ�������ʵ�ʿ�������ѹǿ������������ ��д��һ�����ɣ���
��2���ϳɰ�������������ɼ�����ˮ��Ӧ�Ƶã���Ӧ���Ȼ�ѧ����ʽΪ��
CH4��g��+H2O
 CO��g��+3H2��g������H=+QkJ/mol��Q��0��
CO��g��+3H2��g������H=+QkJ/mol��Q��0����3��һ���¶��£���2L�����з���������Ӧ�������ʵ����ʵ����仯���±�
| ʱ��/min | CH4��mol�� | H20�� mol�� | CO ��mol�� | H2 ��mol�� |
| 0.40 | 1.00 | |||
| 5 | X1 | X2 | X3 | 0.60 |
| 7 | Y1 | Y2 | 0.20 | Y3 |
| 10 | 0.21 | 0.81 | 0.19 | 0.62 |
ǰ5minƽ����Ӧ����v��CH4��= ��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������ٵ�ԭ������� ������ĸ����
a?����CH4 b?�����¶�c?����ѹǿd?����H2
���������¶Ȳ��䣬��1L��������ʼ����0.15mol CH4.0.45mol H2O�� mol CO�� mol H2���ﵽƽ��ʱCH4������ٷֺ������һ��Ͷ����ͬ��
(15 �֣�
�ϳɰ��Ի�ѧ��ҵ������ҵ������Ҫ���塣
(1) ��ϳ����а�1:4�����ʵ���֮�ȳ���N2��H2���а��ĺϳɣ�ͼAΪT0Cʱƽ�������а��������������ѹǿ(P)�Ĺ�ϵͼ��

��д����ҵ�Ϻϳɰ��Ļ�ѧ����ʽ_____________________��
��ͼA�а������������Ϊ15.00%ʱ��H2��ת����=_______ ��
��ͼB��T=5000C���¶�Ϊ4500C��Ӧ��������_______(ѡ����ĸ��a"��b ������ѡ���������______________
����ͼ��֪������ѹǿ�����ԭ�ϵ������ʣ�������ʵ�ʿ�������ѹǿ������������_______(д��һ�����ɣ���
(2) �ϳɰ�������������ɼ�����ˮ��Ӧ�Ƶ�,��Ӧ���Ȼ�ѧ����ʽΪ��
![]()
(3) һ���¶��£���2 L�����з���������Ӧ�������ʵ����ʵ����仯���±�
| ʱ��/min | CH4(mol) | H20( mol) | CO (mol) | H2 (mol) |
| 0 | 0.40 | 1.00 | 0 | 0 |
| 5 | X1 | X2 | X3 | 0.60 |
| 7 | Y1 | Y2 | 0.20 | Y3 |
| 10 | 0.21 | 0.81 | 0.19 | 0.62 |

 CO��g��+3H2��g������H=+QkJ/mol��Q��0��
CO��g��+3H2��g������H=+QkJ/mol��Q��0��