��Ŀ����
 ��֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���ã������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ������ش�
��֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���ã������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ������ش���1����ˮ��Ӧ������ͱ��Ļ�ѧ����ʽΪ
��2������ǿ�Ӧ�����ӷ���ʽ��
��3�������������Ļ�ѧ��������
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
����������ˮ������Һ�к��е������
��4�����õ���B��DΪԭ�ϡ���H+�Ĺ�������������ʣ��Խ�����Ϊ�缫�������ԭ����Ʊ����������ɲ�����ĵ缫��ӦʽΪ
��5����Ӧ���У�0.5molNaClO�μӷ�Ӧʱ��ת��1mol���ӣ��仯ѧ����ʽΪ��
��6��һ�������£�A��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֣���֪�÷�Ӧ����1mol��ʱ�ų�536kJ���������Ȼ�ѧ����ʽΪ��
���㣺������ƶ�
ר�⣺
������A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ��������һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壬���ΪNH3������һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ���ΪN2H4������һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦҲ����ǿ�Ӧ�����о�ˮ���ã�����ΪAl��OH��3�����ת����ϵͼ��֪����Ϊ��������AΪAl��BΪN2����Ϊ��������DΪH2��Ȼ�������ʵ����ʼ���ѧ���������
���
�⣺A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ��������һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壬���ΪNH3������һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18mol���ӣ���ΪN2H4������һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦҲ����ǿ�Ӧ�����о�ˮ���ã�����ΪAl��OH��3�����ת����ϵͼ��֪����Ϊ��������AΪAl��BΪN2����Ϊ��������DΪH2��
��1����Ϊ����������ˮ��Ӧ����Al��OH��3��NH3������ʽΪAlN+3H2O=Al��OH��3+NH3�����ʴ�Ϊ��AlN+3H2O=Al��OH��3+NH3����
��2����Ϊ������������ǿ�Ӧ����Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��3����ΪN2H4������N-H���Թ��ۼ���N-N�Ǽ��Թ��ۼ�����ΪNH3������������ˮ������Һ�к��е������N-H��N��N-H��O��O-H��N��O-H��N��4�������
�ʴ�Ϊ��bc��4��
��4�����õ���N2��H2Ϊԭ�ϡ���H+�Ĺ�������������ʣ��Խ�����Ϊ�缫�������ԭ����Ʊ�NH3��Ӧ������������ԭ��Ӧ���ɣ������ɲ���NH3�ĵ缫��ӦʽΪ6H++2N2+6e-=2NH3���ʴ�Ϊ��6H++2N2+6e-=2NH3��
��5����Ӧ��Ϊ������NaClO�ķ�Ӧ��0.5molNaClO�μӷ�Ӧʱ��ת��1mol���ӣ���ClԪ����+1�۽���Ϊ-1�ۣ��÷�Ӧ����NaCl��ͬʱ����N2H4��ˮ����÷�ӦΪ2NH3+NaClO�TN2H4+NaCl+H2O���ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
��6��-�������£�A��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC������Al��TiO2��C��Ӧ����Al2O3��TiC������1molAl2O3ʱ�ų�536kJ������������2molAl2O3ʱ�ų�536kJ��2=1072�����������Ȼ�ѧ��Ӧ����ʽΪ4Al��s��+3TiO2��s��+3C��s���T2Al2O3��s��+3TiC��s����H=-1072kJ/mol��
�ʴ�Ϊ��4Al��s��+3TiO2��s��+3C��s���T2Al2O3��s��+3TiC��s����H=-1072kJ/mol��
��1����Ϊ����������ˮ��Ӧ����Al��OH��3��NH3������ʽΪAlN+3H2O=Al��OH��3+NH3�����ʴ�Ϊ��AlN+3H2O=Al��OH��3+NH3����
��2����Ϊ������������ǿ�Ӧ����Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��3����ΪN2H4������N-H���Թ��ۼ���N-N�Ǽ��Թ��ۼ�����ΪNH3������������ˮ������Һ�к��е������N-H��N��N-H��O��O-H��N��O-H��N��4�������
�ʴ�Ϊ��bc��4��
��4�����õ���N2��H2Ϊԭ�ϡ���H+�Ĺ�������������ʣ��Խ�����Ϊ�缫�������ԭ����Ʊ�NH3��Ӧ������������ԭ��Ӧ���ɣ������ɲ���NH3�ĵ缫��ӦʽΪ6H++2N2+6e-=2NH3���ʴ�Ϊ��6H++2N2+6e-=2NH3��
��5����Ӧ��Ϊ������NaClO�ķ�Ӧ��0.5molNaClO�μӷ�Ӧʱ��ת��1mol���ӣ���ClԪ����+1�۽���Ϊ-1�ۣ��÷�Ӧ����NaCl��ͬʱ����N2H4��ˮ����÷�ӦΪ2NH3+NaClO�TN2H4+NaCl+H2O���ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
��6��-�������£�A��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC������Al��TiO2��C��Ӧ����Al2O3��TiC������1molAl2O3ʱ�ų�536kJ������������2molAl2O3ʱ�ų�536kJ��2=1072�����������Ȼ�ѧ��Ӧ����ʽΪ4Al��s��+3TiO2��s��+3C��s���T2Al2O3��s��+3TiC��s����H=-1072kJ/mol��
�ʴ�Ϊ��4Al��s��+3TiO2��s��+3C��s���T2Al2O3��s��+3TiC��s����H=-1072kJ/mol��
���������⿼��������ƶϣ����ʵ��ƶ��ǽ����Ĺؼ���������Ϊ������ͻ�ƿڣ�����Ϥ��ѧ�����ʹ������ɣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��֪����˵����ȷ���ǣ�������
| ��ѧʽ | CH3COOH | HClO | H2CO3 |
| Ka | Ka=1.8��10-5 | Ka=3.0��10-6 | K a1=4.1��10-7 K a2=5.6��10-11 |
| A����ͬŨ����CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� |
| B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪ��CO32-+Cl2+H2O�THCO3-+Cl-+HClO |
| C����0.1mol?L-1 CH3COOH��Һ�еμ�NaOH ��Һ��ǡ����ȫ�кͣ�����Ũ�ȴ�С��ϵ��c��Na+ ����c��CH3COO-����c��OH-����c��H+�� |
| D��CO32-��ˮ�ⳣ����K a1�ij˻�ΪKw |
�Ͷ���Ľṹ��ʽΪCH3-CH=CH-COOH�����Т��Ȼ��⣻����ˮ���۴�����Һ���ܶ����������Ը��������Һ���Ը��ݰͶ���Ľṹ�ص㣬�ж���һ�������£�����Ͷ��ᷴӦ�������ǣ�������
| A���ڢܢ� | B���٢ۢ� |
| C���٢ڢۢ� | D���٢ڢۢܢ� |
����������������Ӧ�����Ӧ�õĶ�Ӧ��ϵ��ȷ���ǣ�������
| A������Խǿ�ĺ��������Ƭ��Ӧ��������Խ�� |
| B������ľ�Һ���炙��ʩ�ã���ʹ��Ч���� |
| C��Mg��OH��2��Al��OH��3�����ֽ⣬������������ȼ�� |
| D��ij����ˮ����һ��ʱ�䣬��pH��4.68��Ϊ4.28����Ϊˮ���ܽ��˽϶��CO2 |
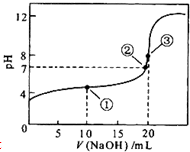 �����£���0.1mol?L-1 NaOH��Һ�ζ�20mL 0.1mol?L-1CH3COOH��Һ�ĵζ�������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���0.1mol?L-1 NaOH��Һ�ζ�20mL 0.1mol?L-1CH3COOH��Һ�ĵζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A�������ʾ��Һ�У�c��Na+����c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� | ||
| B�������ʾ��Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��CH3COOH��+c��OH-�� | ||
| C�������ʾ��Һ�У�c��CH3COO-����c��Na+����c��OH-����c��H+�� | ||
D���������ζ������У���Һ��
|
����0.50mol?L-1��NaOH��Һ245ml�����в�����ȷ���ǣ�������
| A����������ƽ��ȡ4.9gNaOH���� |
| B��NaOH���������ˮ�ܽ⣬Ҫ����Һ��ȴ�����º���ת��������ƿ�� |
| C������ƿ�����ò���ƿ����Ӧ������ƿ�� |
| D������ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��� |
��ѧ���������ճ�������������Ҫ��Ӧ�ã�����˵����ȷ�ǣ�������
| A����������������װ�С���ת��������ʹ�ж���CO��NO��Ӧ����N2��CO2 |
| B����ú�м�������ʯ��ʯ��ʹúȼ�ղ�����SO2��������CaSO3���ɼ��ٶԴ�������Ⱦ |
| C���ߴ��ȵĹ赥�ʹ㷺�����������ά |
| D������ȼ�ջ�ʯȼ���ŷŵķ����к�CO2��SO2���Ӷ�ʹ��ˮ��pH=5.6�γ����� |


