��Ŀ����
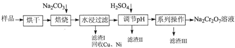
ij�������Ƹ﹤ҵ������CrԪ�صĻ����������ù������£������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe2+��Fe3+��Al3+��Cu2+��Mg2+����
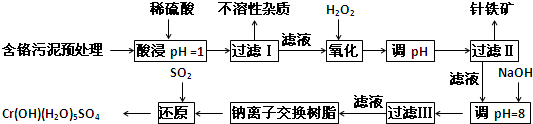
�����²������������������������ʽ����ʱ��Һ��pH������
��1�����ʱ��Ϊ����߽�ȡЧ�ʿɲ�ȡ�Ĵ�ʩ��______������дһ������
��2������H2O2Ŀ��������______���ӣ����йص����ӷ���ʽ______��дһ�֣���������Coethite�����Ե¹�ʫ�˸�£�Coethe�����������ģ����Ԫ����Fe��H��O����ѧʽ��Ϊ89���仯ѧʽ��______��
��3����pH=8��Ϊ�˽�______���ӣ���Fe3+��Al3+��Cu2+��Mg2+��ѡ�������������������ʽ��ȥ���˳��ij����в��ֳ������ܽ�������������������Һ�У����йص����ӷ���ʽ��______��ȡ�����ϲ������Һ��������ͨ��������CO2���������µõ���Ӧ�ij��������йص����ӷ���ʽΪ______��
��4������ƽ���һ����ص�������ԭ����ʽ��
______Na2Cr2O7+______SO2+______H2O=______Cr��OH����H2O��5SO4+______Na2SO4��ÿ����1molCr��OH����H2O��5SO4ʱ���÷�Ӧ��ת�Ƶĵ�����Ϊ______��

�����²������������������������ʽ����ʱ��Һ��pH������
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
| ��ʼ����ʱ��pH | 1.9 | 7.0 | 9.3 | 3.7 | 4.7 | --- |
| ������ȫʱ��pH | 3.2 | 9.0 | 11.1 | 8.0 | 6.7 | 9����9�ܽ⣩ |
��2������H2O2Ŀ��������______���ӣ����йص����ӷ���ʽ______��дһ�֣���������Coethite�����Ե¹�ʫ�˸�£�Coethe�����������ģ����Ԫ����Fe��H��O����ѧʽ��Ϊ89���仯ѧʽ��______��
��3����pH=8��Ϊ�˽�______���ӣ���Fe3+��Al3+��Cu2+��Mg2+��ѡ�������������������ʽ��ȥ���˳��ij����в��ֳ������ܽ�������������������Һ�У����йص����ӷ���ʽ��______��ȡ�����ϲ������Һ��������ͨ��������CO2���������µõ���Ӧ�ij��������йص����ӷ���ʽΪ______��
��4������ƽ���һ����ص�������ԭ����ʽ��
______Na2Cr2O7+______SO2+______H2O=______Cr��OH����H2O��5SO4+______Na2SO4��ÿ����1molCr��OH����H2O��5SO4ʱ���÷�Ӧ��ת�Ƶĵ�����Ϊ______��
��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�ǣ����ӽ�ȡʱ�䡢���Ͻ������������ν�ȡ�ȣ�
�ʴ�Ϊ�������¶ȡ����衢�ӳ���ȡʱ�䡢���˺��ٴν�ȡ��
��2��˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+��Cr3+�л�ԭ�ԣ�Fe2+��Cr3+�ܱ�˫��ˮ����Ϊ�����ӣ��Ա������������ӷ��룬
����Fe2+��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O�����������Ԫ����Fe��H��O����ѧʽ��Ϊ89����1mol��������Ӧ����1molFe����H��O������Ϊ89-56=33����֪Ӧ����2molO��1molH����ѧʽӦΪFeO��OH����
�ʴ�Ϊ��Fe2+��2Fe2++H2O2+2H+=2Fe3++2H2O��FeO��OH����
��3�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������Fe3+���������γ��Ѿ������������NaOH��Һʹ��Һ�ʼ��ԣ���ҺPH=8��Al3+��Cu2+ת��Ϊ������ȥ���˳��ij����в��ֳ������ܽ�������������������Һ�У��漰��ӦΪAl��OH��3+OH-=AlO2-+2H2O��
ȡ�����ϲ������Һ��������ͨ��������CO2���������µõ���Ӧ�ij������漰��ӦΪAlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
�ʴ�Ϊ��Al3+��Cu2+��Al��OH��3+OH-=AlO2-+2H2O��AlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
��4������������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2Cr2O7����Ϊ���ᣬNa2Cr2O7������ԭΪCrOH��H2O��5SO4��ˮ��Һ���������ᷴӦ���������ƣ�����ԭ���غ������д��ƽ����ʽΪNa2Cr2O7+3SO2+11H2O�T2CrOH��H2O��5SO4��+Na2SO4��
�ɷ���ʽ��֪��CrԪ�ػ��ϼ۽���3�ۣ���ÿ����1mol Cr��OH����H2O��5SO4ʱ��ת��3NA���ӣ�
�ʴ�Ϊ��1��3��11��2��1��3NA��
�ʴ�Ϊ�������¶ȡ����衢�ӳ���ȡʱ�䡢���˺��ٴν�ȡ��
��2��˫��ˮ��ǿ�����ԣ���������ԭ�Ե����ʣ�Fe2+��Cr3+�л�ԭ�ԣ�Fe2+��Cr3+�ܱ�˫��ˮ����Ϊ�����ӣ��Ա������������ӷ��룬
����Fe2+��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O�����������Ԫ����Fe��H��O����ѧʽ��Ϊ89����1mol��������Ӧ����1molFe����H��O������Ϊ89-56=33����֪Ӧ����2molO��1molH����ѧʽӦΪFeO��OH����
�ʴ�Ϊ��Fe2+��2Fe2++H2O2+2H+=2Fe3++2H2O��FeO��OH����
��3�������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+������Fe3+���������γ��Ѿ������������NaOH��Һʹ��Һ�ʼ��ԣ���ҺPH=8��Al3+��Cu2+ת��Ϊ������ȥ���˳��ij����в��ֳ������ܽ�������������������Һ�У��漰��ӦΪAl��OH��3+OH-=AlO2-+2H2O��
ȡ�����ϲ������Һ��������ͨ��������CO2���������µõ���Ӧ�ij������漰��ӦΪAlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
�ʴ�Ϊ��Al3+��Cu2+��Al��OH��3+OH-=AlO2-+2H2O��AlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
��4������������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2Cr2O7����Ϊ���ᣬNa2Cr2O7������ԭΪCrOH��H2O��5SO4��ˮ��Һ���������ᷴӦ���������ƣ�����ԭ���غ������д��ƽ����ʽΪNa2Cr2O7+3SO2+11H2O�T2CrOH��H2O��5SO4��+Na2SO4��
�ɷ���ʽ��֪��CrԪ�ػ��ϼ۽���3�ۣ���ÿ����1mol Cr��OH����H2O��5SO4ʱ��ת��3NA���ӣ�
�ʴ�Ϊ��1��3��11��2��1��3NA��

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ