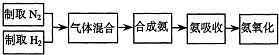
��Ŀ����
ʵ����ģ��ϳɰ��Ͱ����������������£�?

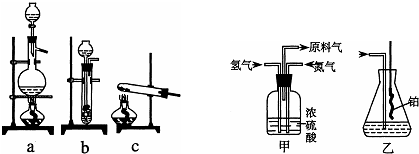
��֪
A��ʵ���ҿ��ñ����������ƣ�NaNO2����Һ�뱥���Ȼ����Һ�����Ⱥ�Ӧ��ȡ������
B����ƿ�еIJ�˿�ǰ���������Ӧ�Ĵ���
��1������ͼ��ѡ����ȡ����ĺ���װ�ã�����______������______��
��2������������ͨ����װ�ã���װ�õ����ó��˽��������⣬������______��______��
��3�����ϳ�����������ȴ����������ͨ����װ�õ�ˮ�����հ���______�����ᡱ���ᡱ������������ԭ����______��
��4������װ������һ��ʱ�䰱����ͨ�������ͬʱ�������ȵIJ�˿������װ�õ���ƿ�ڣ���ʹ��˿���ֺ��ȵ�ԭ���ǣ�______����ƿ�л��ɹ۲쵽�������ǣ�______��
��5��д����װ���а������Ļ�ѧ����ʽ��______��
��6����Ӧ��������ƿ�ڵ���Һ�к���H+��OH-��______��______���ӣ�
�⣺
��1��ʵ���ҿ��ñ����������ƣ�NaNO2����Һ�뱥���Ȼ����Һ�����Ⱥ�Ӧ��ȡ����������ѡ��a��ʵ�������ý���п����ϡ���ᷴӦ��ȡ����������ѡ��װ��b��
�ʴ�Ϊ��a��b��
��2������������ͨ����װ�ã���װ���е�Ũ������Խ��������������ͬʱ���Ը��ݼ�װ����ð���ݵ����������������������ʴ�Ϊ���������壻���������͵��������٣�
��3���ϳɰ��ķ�Ӧ�ǿ���ģ�������һЩ�������������ڣ������Ỻ�ⰱ����������ˮ���µ���ѹ����ᵹ�����ʴ�Ϊ�������Ϊ��������к��д���������ˮ�ĵ����������������壻
��4�����Ĵ�������Ӧ��һ�����ȷ�Ӧ���������ȵIJ�˿������װ�õ���ƿ�ڣ���ʹ��˿���ֺ��ȣ�ͬʱ������������һ���������ױ�Ϊ�������������������Ǻ���ɫ���壬
�ʴ�Ϊ���������Ĵ�������Ӧ��һ�����ȷ�Ӧ���к���ɫ���������
��5�����Ĵ�������Ӧ������һ��������ˮ����4NH3+5O2 4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2 4NO+6H2O��
4NO+6H2O��
��6�����Ĵ�������Ӧ������һ��������ˮ��һ���������ױ�Ϊ��������������������ˮ��Ӧ���������һ����������������Ĵ��ڻ�Ͱ�ˮ��Ӧ��������泥���Һ�л����H+��OH-��NH4+��NO3-���ʴ�Ϊ��NH4+��NO3-��
��������1��������ȡ�����ҩƷ״̬������ѡ����ʵ���ȡ������
��2����װ����ͨ��ð���ݵ���������������������
��3���ϳɰ��ķ�Ӧ�ǿ���ģ�������һЩ�������������ڣ��ݴ����ش�
��4�����Ĵ�������һ�����ȹ��̣�һ�������������ͱ�Ϊ�������������������Ǻ���ɫ���壻
��5�����Ĵ���������һ��������ˮ��
��6��������Һ�������ȷ�����е����ӣ�
������������һ�����ڰ�������ȡ������֪ʶ���ۺ���Ŀ��Ҫ��ѧ�����з����ͽ��������������ۺ���ǿ���Ѷȴ�

 �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�