��Ŀ����
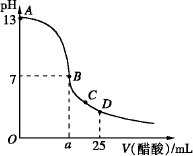
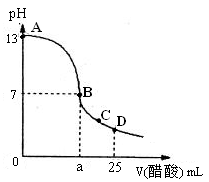
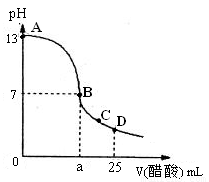
��25 mL����������Һ����μ���0.2 mol��L-1������Һ���ζ���������ͼ��ʾ��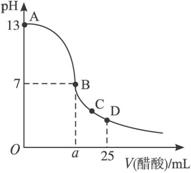
��1��д������������Һ�������Һ��Ӧ�����ӷ���ʽ��__________________________��
��2��������������Һ�����ʵ���Ũ��Ϊ_____________mol��L-1��
��3����B�㣬a_____________12.5 mL������ڡ���С�ڡ����ڡ�����ͬ�������������ȵ��������ƺʹ�����Һ��϶���ǡ�ó����ԣ�����ǰc(NaOH)____________c(CH3COOH)�����ǰ����c(H+)�ͼ���c(OH-)�Ĺ�ϵ��c(H+)_____________c(OH-)��
��4����D�㣬��Һ������Ũ�ȴ�С��ϵΪ_____________��
��1��CH3COOH+OH-====CH3COO-+H2O
��2��0.1
��3������ �� ��
��4��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)
���������⿼��������кͷ�Ӧ������ˮ���Լ�ǿ������ʵĵ����֪ʶ��ǿ������֮��ķ�Ӧ����Ӧ�յ���Һ��pH��7�����Ǵ���7����������Һ�����Ե������Ӧ����CH3COOH������NaOH��Һ��Ũ�ȿ��Ը�����pH�����㡣

��ϰ��ϵ�д�
�����Ŀ