��Ŀ����
��10�֣��Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4��xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
2MnO4����5H2C2O4��6H�� 2Mn2����10CO2����8H2O
����ͬѧ����˵ζ��ķ����ⶨxֵ��
�ٳ�ȡ1.260 g�����ᾧ�壬�������Ƴ�100.00 mLˮ��ҺΪ����Һ��
��ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4
����Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�ŵζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�

�Ʊ�ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x= ��
���ۣ������ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ�� ���ƫ����ƫС�����䡱����ͬ����
�����ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��
2MnO4����5H2C2O4��6H��
����ͬѧ����˵ζ��ķ����ⶨxֵ��
�ٳ�ȡ1.260 g�����ᾧ�壬�������Ƴ�100.00 mLˮ��ҺΪ����Һ��
��ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4
����Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����ﵽ�յ�ʱ����10.00 mL��
��ش�
�ŵζ�ʱ����KMnO4��Һװ����ͼ�е� ����ס����ҡ����ζ����С�

�Ʊ�ʵ��ζ��ﵽ�յ�ı�־��
��ͨ���������ݣ������x= ��
���ۣ������ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ�� ���ƫ����ƫС�����䡱����ͬ����
�����ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��
��10�֣�[ÿ��2��]��1���ף�2�����һ�θ�����ص�����ҺͻȻ������ɫ�����Ϻ�ɫ�����Ұ�����ڲ���ɫ��3��2 ��ƫ�� ��ƫС��
��1��KMnO4��Һ����ǿ�����ԣ��ɸ�ʴ�ܣ���ֻ������ʽ�ζ��ܼ�
��2������KMnO4��Һ����Ϊ��ɫ���ʲ���Ҫʹ��ָʾ���������һ�θ�����ص�����ҺͻȻ�����Ϻ�ɫ���Ұ�����ڲ���ɫ����Ϊ�յ�
��3����2MnO4����5H2C2O4��֪��n(KMnO4)=0.001mol������Һ��n(H2C2O4)=0.0025mol������������H2C2O4Ϊ0.01mol�����������ᾧˮ�����ʵ���Ϊ ���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2
���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2
�����ζ��յ�ʱ���ӵζ��̶ܿȣ������±�Һ�����ƫС�����������H2C2O4����ƫС��ˮ����ƫ�����ɴ˲�õ�xֵ��ƫ��
�����ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�������±�Һ�����ƫ�����������H2C2O4����ƫ��ˮ����ƫС�����ɴ˲�õ�xֵ��ƫС
��2������KMnO4��Һ����Ϊ��ɫ���ʲ���Ҫʹ��ָʾ���������һ�θ�����ص�����ҺͻȻ�����Ϻ�ɫ���Ұ�����ڲ���ɫ����Ϊ�յ�
��3����2MnO4����5H2C2O4��֪��n(KMnO4)=0.001mol������Һ��n(H2C2O4)=0.0025mol������������H2C2O4Ϊ0.01mol�����������ᾧˮ�����ʵ���Ϊ
 ���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2
���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2�����ζ��յ�ʱ���ӵζ��̶ܿȣ������±�Һ�����ƫС�����������H2C2O4����ƫС��ˮ����ƫ�����ɴ˲�õ�xֵ��ƫ��
�����ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�������±�Һ�����ƫ�����������H2C2O4����ƫ��ˮ����ƫС�����ɴ˲�õ�xֵ��ƫС

��ϰ��ϵ�д�
 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д� ��������ϵ�д�
��������ϵ�д�
�����Ŀ
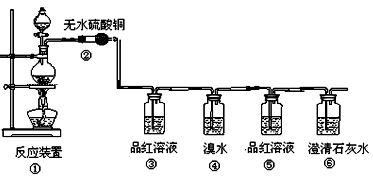
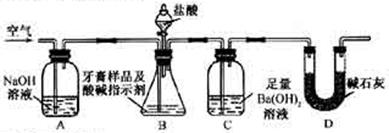
 ��ijƷ��������Ħ�����ɷּ��京����������̽����
��ijƷ��������Ħ�����ɷּ��京����������̽���� __________________________________________��
__________________________________________��
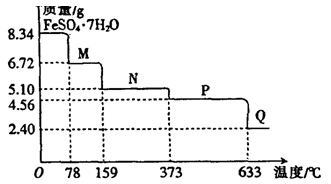
 ___��
___��

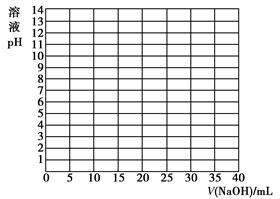
 ��Һ��������һ�ݷ��ڿ�����һ��ʱ�����Һ��pH ���������С�����䡱����ԭ���� ������֪Ũ�ȵ������к�����������Һ�����кͷ��ڿ�����һ��ʱ�����Ƿ���Һ������������Ϊ
��Һ��������һ�ݷ��ڿ�����һ��ʱ�����Һ��pH ���������С�����䡱����ԭ���� ������֪Ũ�ȵ������к�����������Һ�����кͷ��ڿ�����һ��ʱ�����Ƿ���Һ������������Ϊ ���к���һ����Һ������������Ϊ
���к���һ����Һ������������Ϊ ����1���Լ���Ϊָʾ��
����1���Լ���Ϊָʾ��