��Ŀ����
13������һ���Σ���A��B��C��D��E����Ԫ����ɣ����������Ƕ�����Ԫ�أ�������ˮ��ɵ�����������ӣ����к�����A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���壮�ü�������ʵ�飺
��ȡ�����ľ�����������ˮ�����Һ��
��ȡ��������Һ���Թ��е���KSCN��Һ���������ٵ�����ˮ����Һ�ʺ�ɫ��
��ȡ��������Һ���Թ��м�������NaOH��Һ�����ȣ�
��ȡ��������Һ���Թ��У������м���ϡ���ᣬ�ټ���BaCl2��Һ�����ְ�ɫ������
�ش��������⣺
��1��C��Ԫ�ط�����S��D�����ڱ��е�λ�õ������ڵڢ��壮
��2�������ӷ���ʽ��ʾʵ����������ԭ��2Fe2++Cl2=2Cl-+2Fe3+��Fe3++3SCN-?Fe��SCN��3��
��3��ʵ����г��ֵ��������а�ɫ��״�������ɣ�Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ�������д����̼�����ζ�������ɣ�
��4�����������������������ʵ���֮��Ϊ3��2����Ļ�ѧʽΪ��NH4��2Fe��SO4��2��
��5��ijͬѧ�����Ͷ�����̼Ϊԭ���Ʊ�̼����泥������ķ�����B��
A���Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백����
B���Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼��
�����������NH4+�ķ���Ϊȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������NH4+��
���� ��A��B�γɵ�10���������ӣ�BԪ����һ�ֺ���û�����ӣ������Ƴ�B�϶���H����ôA�϶���N������Һ�к���NH4+��AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���壬D��S��E��O��ȡ��������Һ���Թ��е������軯����Һ���������ٵ��백ˮ����Һ�ʺ�ɫ�������Һ�϶�����Fe2+��ȡ��������Һ���Թ��У������м���ϡ���ᣬ�ټ����Ȼ�����Һ�����ְ�ɫ�����������Һ�к���SO42-����������������Һ�к���Fe2+��SO42-��NH4+�������������������ʵ���֮��Ϊ3��2���Ļ�ѧʽ�����ǣ�NH4��2Fe��SO4��2���պþ����Ħ������Ϊ392g/mol�����Լ���Ļ�ѧʽΪ��NH4��2Fe��SO4��2•6H2O���Դ˽����⣮
��� �⣺��A��B�γɵ�10���������ӣ�BԪ����һ�ֺ���û�����ӣ������Ƴ�B�϶���H����ôA�϶���N������Һ�к���NH4+��AԪ��ԭ�Ӻ�����������E����1��D��E����ͬ���壬D��S��E��O��ȡ��������Һ���Թ��е������軯����Һ���������ٵ��백ˮ����Һ�ʺ�ɫ�������Һ�϶�����Fe2+��ȡ��������Һ���Թ��У������м���ϡ���ᣬ�ټ����Ȼ�����Һ�����ְ�ɫ�����������Һ�к���SO42-����������������Һ�к���Fe2+��SO42-��NH4+����
��1�������Ϸ�����֪D��S��C��Fe��λ�����ڱ��������ڵڢ��壬
�ʴ�Ϊ��S���������ڵڢ��壻
��2������Һ�к���Fe2+��������ˮ����2Fe2++Cl2=2Cl-+2Fe3+������Fe3+������KSCN����Fe3++3SCN-?Fe��SCN��3����Һ��ɺ�ɫ��
�ʴ�Ϊ��2Fe2++Cl2=2Cl-+2Fe3+��Fe3++3SCN-?Fe��SCN��3��
��3��ȡ��������Һ���Թ��м�������NaOH��Һ������Fe2++2OH-=Fe��OH��2�����а�ɫ��״�������ɣ�
��������4Fe��OH��2+O2+2H2O=4Fe��OH��3��Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ��ͬʱ�ְ������ɣ�
�ʴ�Ϊ���а�ɫ��״�������ɣ�Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ�������д����̼�����ζ�������ɣ�
��4������Һ�к���Fe2+��SO42-��NH4+�������������������ʵ���֮��Ϊ3��2���Ļ�ѧʽ�����ǣ�NH4��2Fe��SO4��2��
�ʴ�Ϊ����NH4��2Fe��SO4��2��
��5�����ڰ�����ˮ�е��ܽ��ԶԶ���ڶ�����̼�������������Ͷ�����̼Ϊԭ���Ʊ�̼�����ʱӦ�Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼����ѡB�������������NH4+�ķ���Ϊȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������NH4+��
�ʴ�Ϊ��B��ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������NH4+��
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע����ݷ�Ӧ�������ж����ʣ�ѧϰ��ע�������ػ���֪ʶ��

| A�� | 0.4 mol•L-1 | B�� | 0.3 mol•L-1 | C�� | 0.2 mol•L-1 | D�� | 0.1 mol•L-1 |
| A�� | 20 mL 0.5 mol/L AlCl3��Һ | B�� | 30 mL 1 mol/L KCl��Һ | ||
| C�� | 7.5 mL 2 mol/L MgCl2��Һ | D�� | 10 mL 3 mol/L NaCl��Һ |
| A�� | ��ۻ��Dz��� | B�� | ��Һû�ж�������� | ||
| C�� | ���ȶ� | D�� | ��ɢ������ֱ������1��10-7m |
| A�� | Na2S | B�� | NaHSO4 | C�� | SO2 | D�� | H2SO4 |
| A�� | ��A��ʾ�ķ�Ӧ������0.4mol•L-1•min-1 | |
| B�� | �ֱ���B��C��D��ʾ��Ӧ�����ʣ������3��4��1 | |
| C�� | ��2minĩʱ�ķ�Ӧ���ʣ��÷�Ӧ��B����ʾ��0.3mol•L-1•min-1 | |
| D�� | ����2min����A��C��ʾ�ķ�Ӧ���ʵ�ֵ������ͬ�� |
 ��Һ������Զ������������Ҫ��Ӱ�죮
��Һ������Զ������������Ҫ��Ӱ�죮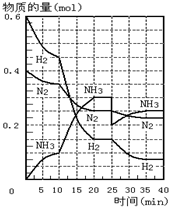 500�桢20MPaʱ����H2��N2����һ�ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ������H2��N2��NH3���ʵ����仯��ͼ��ʾ��������˵����ȷ���ǣ�������
500�桢20MPaʱ����H2��N2����һ�ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ������H2��N2��NH3���ʵ����仯��ͼ��ʾ��������˵����ȷ���ǣ�������