��Ŀ����
�������ʵ�顱���м��ס����㡢��Լ����ɫ���ŵ㣬�۲������ĸ������ʵ�顱�����г�װ��δ���������ж�����˵����ȷ���ǣ�����������

����12�֣�����Y��������������Ͽ��Խ�������ʵ�飨�̶�װ���ԣ����������ش��������⣺

| A������ʱ�����ϲ��㼯�˹���NH4Cl��˵��NH4Cl�����ȶ��ԱȽϺ� |
| B������ʱ���ڡ�����Һ����죬��ȴ���ֶ���Ϊ��ɫ |
| C�����У���������ˮ�е�������������ɫ��������ڱ�ˮ�е�������������ɫ��dz |
| D���ĸ������ʵ�顱���������Ļ�ѧ��Ӧ���ǿ��淴Ӧ |
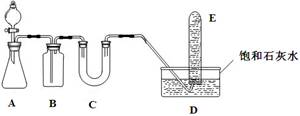
| ��1�� |  | ʵ��Ŀ�ģ���֤SO2�������� ����ͷ�ι���Ũ����ֱ����Y�ܵ�����֧���У���֧�ܽ��洦ʵ������Ϊ________________������������ˮ��Ŀ����___________________�� |
| ��2�� |  | ʵ��Ŀ�ģ�̽��SO2��BaCl2��Ӧ�������������� ��������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ�Y����Ӧ���õ�ҩƷ��_________��_________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ____________�� |
| ��3�� |  | ʵ��Ŀ�ģ�п���Ͻ����������IJⶨ �ٶ�ȡ������������ʱ��������ˮ���е�Һ�������������Һ�棬Ӧ��ȡ�Ĵ�ʩ��__________ _________________________________________��  �����Ƶ�п���Ͻ������Ϊ0.117g���������г�����Ϊ1. 00mL��ĩ����Ϊ45.80mL����Ͻ������ĺ���Ϊ________%������2λС�����������������ɱ�״���µ���ֵ���� �����Ƶ�п���Ͻ������Ϊ0.117g���������г�����Ϊ1. 00mL��ĩ����Ϊ45.80mL����Ͻ������ĺ���Ϊ________%������2λС�����������������ɱ�״���µ���ֵ���� |

��

��ϰ��ϵ�д�
�����Ŀ
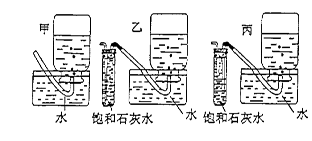

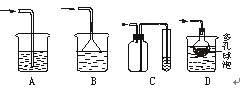
 ������Ũ��ʹNaCl���������������������������˳���ź������ǡ���
������Ũ��ʹNaCl���������������������������˳���ź������ǡ���