��Ŀ����
5����ͼ��ʾ�������̶�������������������ƶ���M��N���������о�������Ӧ��3A��g��?xB��g��+xC��g����H=-192kJ•mol-1����M��N�ж�ͨ��3mol A �����壬��ʼM��N�ݻ���ͬ�������¶Ȳ��䣮����˵����ȷ���ǣ�������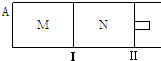
| A�� | ��x=2���ﵽƽ���B �����������ϵΪ���գ�M�����գ�N�� | |
| B�� | ��x��2���ﵽƽ���B��ת���ʹ�ϵΪ������M��������N�� | |
| C�� | ��x��2��C��ƽ�����ʵ���Ũ�ȹ�ϵΪ��c��M����c��N�� | |
| D�� | x����Ϊ��ֵ����ʼʱ��N�����г�������ֵ��A��ƽ���N������B��Ũ�Ⱦ���� |
���� M�����Ǻ��º����½�����ƽ�⣬N�����Ǻ��º�ѹ�½�����ƽ�⣬
A����x=2�����ڷ�Ӧ�������������M������ѹǿ����N��
B����x��2�����ڷ�Ӧ�����������С��N����������ƽ���൱�ں��º����½�����ƽ���С���������ѹǿ����ƽ�������ƶ���
C����x��2�����ڷ�Ӧ�������������N����������ƽ���൱�ں��º����½�����ƽ���������������ѹǿ��С��ƽ�������ƶ���
D�����º�ѹ�£�x����Ϊ��ֵ����ʼʱ��N�����г�������ֵ��A��һ�ߵ���B��C�����ʵ���֮�ȶ���1��1��Ϊ��Чƽ�⣬����ֵ�Ũ����ͬ��
��� �⣺M�����Ǻ��º����½�����ƽ�⣬N�����Ǻ��º�ѹ�½�����ƽ�⣬
A����x=2�����ڷ�Ӧ�������������M������ѹǿ����N��ѹǿ����ƽ�������ƶ����ﵽƽ���B �����������ϵΪ���գ�M�����գ�N������A����
B����x��2�����ڷ�Ӧ�����������С��N����������ƽ���൱�ں��º����½�����ƽ���С���������ѹǿ����ƽ�������ƶ����ﵽƽ���B��ת���ʹ�ϵΪ������M��������N������B����
C����x��2�����ڷ�Ӧ�������������N����������ƽ���൱�ں��º����½�����ƽ���������������ѹǿ��С��ƽ�������ƶ���C��ƽ�����ʵ���Ũ�ȹ�ϵΪ��c��M����c��N������C����
D�����º�ѹ�£�x����Ϊ��ֵ����ʼʱ��N�����г�������ֵ��A��һ�ߵ���B��C�����ʵ���֮�ȶ���1��1��Ϊ��Чƽ�⣬����ֵ�Ũ����ͬ����D��ȷ��
��ѡD��
���� ���⿼��ѧ��Ӱ�컯ѧƽ���ƶ������أ�ע����º����µ�ƽ������º�ѹ�µ�ƽ���Ĺ�ϵ�Լ���Чƽ����жϣ����Ը�����ѧ֪ʶ���ش��Ѷ��еȣ�

| A�� | pH=4��NaHSO3��Һ�У�c��Na+ ����c��HSO3-����c��H2SO3����c��SO32-�� | |
| B�� | 0.01mol•L-1 ��NaHCO3 ��Һ�д�������ƽ�⣺HCO3-?H++CO3 2-����ˮϡ����Һ�е�HCO3-��H+��CO3 2-Ũ�ȼ��� | |
| C�� | Ũ�Ⱦ�Ϊ0.1mol•L-1 ��CH3COOH��CH3COOK�����Һ�У�2c��H+��+c��CH3COOH��=2c��OH-��+c��CH3COO-�� | |
| D�� | ��Ka��HA��=3.6��10-4��Ka��HB��=1.75��10-5��������ʵ���Ũ�ȵ�NaA��KB��Һ��ȣ�c��Na+ ��-c��A-��=c��K+ ��-c��B-�� |
| A�� | 7�� | B�� | 8�� | C�� | 9�� | D�� | 10�� |
| ʵ�������� | �����¼ | ���۽��� | |
| A | ������Ũ������μ���Cu ��ϡ����Ļ������ | ��������ɫ���� | ���ᱻ��ԭΪNO2 |
| B | ������ǯ��ס�����ھƾ��� �ϼ��� | �����ۻ����������� | �۵㣺Al2 O3��Al |
| C | ��ij�Ȼ�������Һ�м��� Na2O2��ĩ | ���ֺ��ɫ���� | ����Na2O2��ĩǰ��ԭ�Ȼ� ������Һ�Ѿ����� |
| D | ����ɫʯ����Һ�г�����ʱ ��ͨ������ | ��Һ�ȱ�죬���� Ϊ��ɫ | ������Ư���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��״���£�2.24L�����еķ�����Ϊ0.1nA | |
| B�� | 25��ʱ��PH=13��Ba��OH��2����Һ�к���OH-����ĿΪ0.2nA | |
| C�� | 1L0.1mol•L-1Al2��SO4 ��3����ҺAl3+����ĿΪ0.2nA | |
| D�� | 1.5molNO2������H2O��Ӧ��ת�Ƶĵ�����ΪnA |
| A�� | pH=4.5�ķ���֭��c��H+����pH=6.5��ţ����c��H+����100�� | |
| B�� | ��ͬŨ�Ⱥ������ǿ����ǿ����Һ��Ϻ���Һ��PH=7 | |
| C�� | ��NaAlO2��Һ�еμ�NaHCO3��Һ���г������������� | |
| D�� | ����Ӧ2A��g��+B��g��=2C��g����һ����������һ���Է��ķ�Ӧ����÷�ӦΪ���ȷ�Ӧ |

 ��Ի�ѧ��Ӧ�е������仯����������⣮
��Ի�ѧ��Ӧ�е������仯����������⣮