��Ŀ����
14�� ��֪A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ����ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��֪A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ����ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ��Իش��������⣺
��1����̬Gaԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p63d104s24p1��
��2��A��B��C�ĵ�һ�������ɴ�С��˳��N��C��Si����Ԫ�ط��ű�ʾ����
��3��BԪ�صĵ��ʷ�������2���м������以Ϊ�ȵ���������ʵĻ�ѧʽ����ΪCO��CN-����дһ�֣���
��4������A���������������ԭ�Ӳ�ȡsp�ӻ����侧���������������Ϊ���Ӽ���������
��5��EH3���ӵĿռ乹��Ϊ�����Σ���е���BH3��ȵͣ���ߡ��͡�����ԭ��
��NH3����֮��������
��6����CuSO4��Һ����μ���BH3��ˮ��Һ����������ɫ��������������ܽ�õ�����ɫ������Һ����д�������ܽ�����ӷ���ʽCu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
���� A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬����Χ�����Ų�ʽΪns2np3����BΪNԪ�ء�DΪPԪ�ء�EΪAs��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ����侧���ṹ��֪ΪCO2��AΪ̼Ԫ�أ�ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮����֪CΪSi��
��� �⣺A��B��C��D��E�������ڱ��е�ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ���������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ����״̬����Χ�����Ų�ʽΪns2np3����BΪNԪ�ء�DΪPԪ�ء�EΪAs��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ����侧���ṹ��֪ΪCO2��AΪ̼Ԫ�أ�ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ��뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮����֪CΪSi��
��1��Ga��Alͬ�������ڣ�Gaԭ�Ӻ��������Ϊ13+18=31����������Ų�ʽΪ��1s22s22p63s23p63d104s24p1���ʴ�Ϊ��1s22s22p63s23p63d104s24p1��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ�ͬ�������϶��µ�һ���������ʵ�һ�����ܣ�N��C��Si��
�ʴ�Ϊ��N��C��Si��
��3��BԪ��ΪN2���ṹʽΪN��N����������2���м������以Ϊ�ȵ���������ʵĻ�ѧʽ����ΪCO��CN-��
�ʴ�Ϊ��2��CO��CN-��
��4������A��������ΪCO2��Ϊֱ���νṹ��������Cԭ�Ӳ�ȡsp�ӻ������ڷ��Ӿ��壬�侧���������������Ϊ���Ӽ���������
�ʴ�Ϊ��sp�����Ӽ���������
��5��AsH3������NH3���ӹ������ƣ�Ϊ�����νṹ������NH3����֮������������AsH3�ķе��NH3�ĵͣ�
�ʴ�Ϊ�������Σ��ͣ�NH3����֮����������
��6����CuSO4��Һ����μ���NH3��ˮ��Һ��������Cu��OH��2��ɫ��������������ܽ�õ�����ɫ������Һ������[Cu��NH3��4]2+�������ܽ�����ӷ���ʽ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
�ʴ�Ϊ��Cu��OH��2+4NH3•H2O=[Cu��NH3��4]2++2OH-+4H2O��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰�ṹ�������ʹ�ϵ����������Ų��������ܡ���ѧ�����ȵ����塢�ռ乹�����ӻ���ʽ�жϡ�����������ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱�֪ʶ�Ļ�����

 ���±���Ԫ�����ڱ��е�ǰ�����ڣ��١���Ϊ��Ӧ��Ԫ�أ������ѡ����ʵ�Ԫ�ػش�����
���±���Ԫ�����ڱ��е�ǰ�����ڣ��١���Ϊ��Ӧ��Ԫ�أ������ѡ����ʵ�Ԫ�ػش����� | �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | �� | |||||||||||||||
��2���ڡ�����Ԫ���γɵĻ�����Ŀռ乹��Ϊƽ���������Σ�������ԭ�ӵ��ӻ�����Ϊsp2��
��3��д��Ԫ�آ��̬ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d104s1��
��4���Ƚϣ���һ�����ܣ��ۣ��ܣ��縺�ԣ��ܣ��ݣ������������=������
��5��Ԫ�آ���CO���γɵ�X��CO��5�ͻ�����û����ﳣ���³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��жϸû����ᄃ�����ڷ��Ӿ��壨������ͣ���
��6��Ԫ�آ�����ӵ��������ﲻ����ˮ���������ڰ�ˮ�У���������NH3���ϵ�������Ϊ��λ����
��7�������ߵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ��������������������������������ʵ�ʺ��е�ԭ�Ӹ���֮��Ϊ2��1��
������ͭ˿����Ũ���ᣬ���ȣ�
������������ɫ����������ʱ�����ͭ˿��ֹͣ���ȣ�
����ȴ�ӷ�Ӧ��Ļ�����з������ɫ������ϴ�������ﱸ�ã�
�ش��������⣺
��1��������������Ļ�ѧʽΪSO2��
��2������ Cu2+��Һ�еμ�K4[Fe��CN��6]��Һ���ܲ������ɫ�������ֽ�������ɫ��������ϡ�����У�������Ժ��ٵμ�K4[Fe��CN��6]��Һ��δ�����ɫ�������ɴ����ý����Ǻ�ɫ�����в�����CuO��
��3��Ϊ֤����ɫ��������ͭ�������������ʵ�飺
| װ�� | ���� | ���ۼ����� |
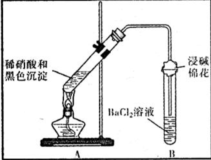 | ��A�Թ��к�ɫ�������ܽ� ��A�Թ��Ϸ����ֺ���ɫ���� ��B�Թ��г��ְ�ɫ���� | a�������˵����ɫ�������� ��ԭ���ԣ� b���Թ�B�в�����ɫ�������ܷ�Ӧ�����ӷ���ʽΪ NO2+SO2+Ba2++H2O�TBaSO4��+NO��+2H+ |
��5��Ϊ�ⶨ��ɫ������Cu2S �İٷֺ�����ȡ0.2g ��������ú�ɫ��������������Һ���� 40.0mL 0.075mol/L KMnO4��Һ������������Ӧ���£�
8MnO4-+5Cu2S+44H+�T10Cu2++5SO2��+8Mn2++22H2O
6MnO4-+5CuS+28H+�T5Cu2++5SO2��+6Mn2++14H2O
��Ӧ�������Һ���Ͼ�SO2�������ĸ��������Һǡ����35.0mL 0.1mol/L ��NH4��2Fe��SO4��2 ��Һ��Ӧ��ȫ����������Cu2S ����������Ϊ40%��
| A | B | |
| C | D |
��1��Aλ��Ԫ�����ڱ������ڣ�VA�壬���⻯��ķ���ʽ��NH3��
��2�����������У���ȷ����a������ĸ����
a���ȶ��ԣ�A���⻯�C���⻯�� b����ԭ�ԣ�B2-��D2-
c�����ԣ�H4CO4��H2DO4 d������ϼ�ֵ��D=B��A��C
��3��DB2ͨ�����й������̿��ƻ���ҵԭ��H2DB4�������ԴH2��

��ԭ�����DB2���뷴Ӧ�ĵ缫Ϊ������д���缫��ӦʽSO2+2H2O-2e-=4H++SO42-������5mol DB2�μӷ�Ӧ��������Ӧ������ģ��������ɱ�״����112L H2��
��Ϊ����������ķ���Ч����ȡ������H2DB4��Һ���Թܣ���������μ���AgNO3��Һ����ַ�Ӧ�����۲쵽����ɫ�����������������ɰ�ɫ������֤������Ч���Ϻã�
�۽��ù����������ܷ�Ӧ�Ļ�ѧ����ʽ��ʾΪ��SO2+2H2O=H2SO4+H2��
| A�� | $\underset{\stackrel{\;}{C}}{•}$H2O | B�� | $\underset{\stackrel{\;}{N}}{•}$2H4 | C�� | $\underset{\stackrel{\;}{B}}{•}$F3 | D�� | $\underset{\stackrel{\;}{C}}{•}$2H4 |
| A�� | ʹ��ת��Ϊ���ȶ����ʣ����ڱ�������� | |
| B�� | ��ǿƯ���������� | |
| C�� | ʹת��Ϊ��������ˮ������ | |
| D�� | �����ȵ�����������������Ư�ס����� |
| A�� | ��-���屽ֻ��һ�� | |
| B�� | ������������ԭ����ͬһƽ���� | |
| C�� | ���ױ�û��ͬ���칹�� | |
| D�� | �����ܷ����ӳɷ�ӦҲ�ܷ���ȡ����Ӧ |
 ��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�
��֪X��Y��Z��J��Q���ֶ���������Ԫ�أ�ԭ��������������Ԫ��Z�ڵؿ��к�����ߣ�JԪ�ص���ɫ��Ӧ�ʻ�ɫ��Q�������������������������Ϊ3��8��X����J�γ����ӻ������J+�İ뾶����X-�İ뾶��Y2�ǿ�����Ҫ�ɷ�֮һ����ش�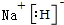
 ��ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ���21���ͻ�õ��ܵ���Ҫ;���������꿪���ļ״�ȼ�ϵ���Dz��ò����缫����������е����ӽ���Ĥֻ�������Ӻ�ˮ����ͨ�����乤��ԭ����ʾ��ͼ��ͼ��ʾ��
��ѧ��Ԥ�ԣ�ȼ�ϵ�ؽ���21���ͻ�õ��ܵ���Ҫ;���������꿪���ļ״�ȼ�ϵ���Dz��ò����缫����������е����ӽ���Ĥֻ�������Ӻ�ˮ����ͨ�����乤��ԭ����ʾ��ͼ��ͼ��ʾ��