��Ŀ����
16�������£������йش�����Һ�������в���ȷ���ǣ�������| A�� | pH=4.3��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� | |
| B�� | Ũ��Ϊ0.2mol/L��CH3COOH��Һ��ũ��Ϊ0.1mol/L��NaOH��Һ�������Ϻ�c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��] | |
| C�� | ������Һ������ˮϡ�ͣ�$\frac{c��C{H}_{3}COOH��}{{c}^{2}��{H}^{+}��}$�������� | |
| D�� | amol/LCH3COOH��Һ��bmol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CH3COO-������һ����a��b |
���� A����Һ�����ԣ���c��H+����c��OH-�������ݵ���غ���������
B��Ũ��Ϊ0.2mol/L��CH3COOH��Һ��Ũ��Ϊ0.1mol/L��NaOH��Һ�������Ϻ��Ũ�Ⱦ�Ϊ0.05mol/L��CH3COOH��CH3COONa�Ļ������������غ�͵���غ���������
C��������Һ�м�����ˮϡ�ͣ�����ƽ�ⳣ�����䣬����Һ�е�c��H+��=c��CH3COO-����
D��amol/LCH3COOH��Һ��bmol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CH3COO-��������ݵ���غ��֪��c��H+����c��OH-�����ݴ˷�����
��� �⣺A�����ݵ���غ��֪��c��H+��+c��Na+��=c��OH-��+c��CH3COO-��������Һ�����ԣ���c��H+����c��OH-�����ʿ�֪c��Na+����c��CH3COO-������A��ȷ��
B��Ũ��Ϊ0.2mol/L��CH3COOH��Һ��Ũ��Ϊ0.1mol/L��NaOH��Һ�������Ϻ��Ũ�Ⱦ�Ϊ0.05mol/L��CH3COOH��CH3COONa�Ļ������������غ��֪��
c��CH3COOH��+c��CH3COO-��=2c��Na+�� ��
c��CH3COO-��+c��OH-��=c��Na+��+c��H+�� ��
���ڡ�2-�ٿɵã�c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��]����B��ȷ��
C��������Һ�м�����ˮϡ�ͣ�����ƽ�ⳣ��K=$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$���䣬����Һ�е�c��H+����c��CH3COO-������K=$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$��$\frac{{c}^{2}��{H}^{+}��}{c��C{H}_{3}COOH��}$�������䣬��$\frac{c��C{H}_{3}COOH��}{{c}^{2}��{H}^{+}��}$�������䣬��C��ȷ��
D��amol/LCH3COOH��Һ��bmol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CH3COO-��������ݵ���غ��֪c��H+����c��OH-��������Һ�Լ��ԣ���b=aʱ������ǡ����ȫ��Ӧ���ɴ����ƣ���Һ�Լ��ԣ���b��aʱ��NaOH��Һ��������Һ�Լ��ԣ�����a������b������ֻ�Ǽ���������ʱ����ҺҲ�����Լ��ԣ��ʲ�һ����a��b����D����
��ѡD��
���� ���⿼��������Һ�е�����Ũ�ȵĴ�С�Ƚ��Լ��жϣ���������Һ�е������غ㡢����غ�����ʵ���Ũ�ȹ�ϵ���жϣ���Ŀ�Ѷ��еȣ�

| A�� | ����������������ʹƷ����Һ��ɫ�����ǵ�Ư��ԭ����ͬ | |
| B�� | �����ľ�������ִ���ѧ��������Ʒ�Ļ���ԭ�� | |
| C�� | �ö����ЧӦ�ɼ���FeCl3��Һ��Fe��OH��3���� | |
| D�� | NH3��ˮ��Һ���Ե��磬����NH3�ǵ���� |
| ѡ�� | ����ͨ��������Һ�� | ʵ������ | ���� |
| A | ����KSCN��FeCl2��Һ | ��� | �������л�ԭ�� |
| B | ���з�̪��NaOH��Һ | ��ɫ | ��������Ư���� |
| C | ��ɫʯ����Һ | �ȱ�����ɫ | �����������ԡ�Ư���� |
| D | ��������ͨ�����ˮ�� | ��ҺpH��7 ��dz����ɫ | ������ˮ��Ӧ�����������ʣ� �Ҹ÷�ӦΪ���淴Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ѧ����50mL��Ͳ��ȡ46.70mLŨ���� | |
| B�� | ��ѧ���ù㷺pH��ֽ�ⶨ��Һ������ԣ�pH=14.5 | |
| C�� | ��ѧ����NaOH��Һ���õ�����ƽ��ȡ����1.220g | |
| D�� | ��ѧ���ú���ζ�����ȡ25.00mL0.1mol/L������ |
 ���ӵ�������Һ����ÿ����Һ�зֱ���������������ƹ��塢����Ũ���ᣬ�������Ը��������Һ����������Ŀ�����ٵ��ǣ�������
���ӵ�������Һ����ÿ����Һ�зֱ���������������ƹ��塢����Ũ���ᣬ�������Ը��������Һ����������Ŀ�����ٵ��ǣ�������| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢� | D�� | �ۢݢ� |
��1����һ�������£����淴ӦmA?nB+pC��H���ﵽƽ��״̬��
����A��B��C�������壬����ѹǿ��ƽ��������Ӧ�����ƶ�����m����n+p������ڡ�����С�ڡ����ڡ�����
�������������䣬���Ⱥ�A��������С����Ӧ��H����0������ڡ�����С�ڡ����ڡ�����
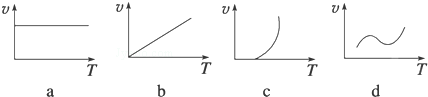
��2��ijЩ�����������FeXOY����ĩ��Al����þ������ȼ�¿��Է������ȷ�Ӧ�����з�Ӧ���ʣ�v�����¶ȣ�T���Ĺ�ϵʾ��ͼ�������ȷ�Ӧ��ӽ�����c
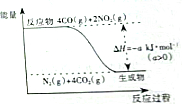
��3��һ���¶��£�������Ӧ��FeO��s��+CO��g��?Fe��s��+CO2��g����H����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
| �¶�/�� | 1000 | 1100 |
| ƽ�ⳣ�� | 0.68 | 0.50 |
�ٸ÷�Ӧ�ġ�H��0�����������������=������
��T��ʱ����FeO��s����CO��g����3.0mol����10L���ܱ������У���Ӧ�ﵽƽ����COת����ΪW1��c��CO2��=0.15mol•L-1�����¶�T���ڣ�����ڡ��������ڡ��������ڡ���1000������ʱ�����������������ٳ���2.0mol CO��g�����ٴ�ƽ��ʱ���COת����ΪW2����W1=W2�����������������=������
 �о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���壮
�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���壮 �ҹ���ʷ�ƾã�������������̴��ͻ�ұ��ͭ�����ڳ����ĸ���ͭ�����涼Ϊ��ɫ������ѧ�Ҽ��飬��Щͭ����Ϊ��ʽ̼��ͭ[Cu2��OH��2CO3]��
�ҹ���ʷ�ƾã�������������̴��ͻ�ұ��ͭ�����ڳ����ĸ���ͭ�����涼Ϊ��ɫ������ѧ�Ҽ��飬��Щͭ����Ϊ��ʽ̼��ͭ[Cu2��OH��2CO3]��