��Ŀ����
��800��ʱ�����п��淴Ӧ��![]()
![]()
![]() ������ʼŨ��
������ʼŨ��![]() Ϊ2mol/L��
Ϊ2mol/L��![]() Ϊ3mol/L����Ӧ��ƽ��ʱ��CO��ת����Ϊ60%�����
Ϊ3mol/L����Ӧ��ƽ��ʱ��CO��ת����Ϊ60%�����![]() ��ʼŨ��������6mol/L����CO��ת����Ϊ75%��
��ʼŨ��������6mol/L����CO��ת����Ϊ75%��![]() ��ʼŨ�ȵĸı��COת���ʳ��ֹ����Եı仯��
��ʼŨ�ȵĸı��COת���ʳ��ֹ����Եı仯��
��800��ʱ����ʼ![]() Ϊamol/L����ʼ
Ϊamol/L����ʼ![]() Ϊbmol/L����Ӧ�ﵽƽ��ʱ
Ϊbmol/L����Ӧ�ﵽƽ��ʱ![]() Ϊcmol/L��
Ϊcmol/L��
(1)��a=2mol/L��b=8mol/Lʱ���ﵽƽ��ʱCO��ת����Ϊ________��
(2)��a=5mol/L��![]() ʱ��bΪ________��
ʱ��bΪ________��
(3)��a=bʱ��c/a����________��
(4)���Ա�ʾa��b��c֮���ϵ�Ĵ���ʽ��________��
�𰸣�
������
������
|
(1) (2) ��
(3) (4) |

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ
�ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɶ����ѵ����ȼ�ϣ�
��ش��������⣺
��1����һ�ܱ������н��з�ӦCH4��g��+H2O��g��?CO��g��+3H2��g����H1=+206.1kJ/mol�����CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ����Ӧ���е�ǰ5min�ڣ�v��H2��=______��10minʱ���ı���������������______��
��2����ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȣ�����ͬ�¶��·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g����H2=+247.3kJ/mol����ά�ַ�Ӧ�������¶Ȳ��䣮��֪��������CH4��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��
��3����ӦCO��g��+H2O��g��?CO2��g��+H2��g����H3=-41.2kJ/mol��800��ʱ�Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
��ʱ��Ӧ�������淴Ӧ���ʵĹ�ϵʽ��______������ţ���
a��v��������v���棩 b��v��������v���棩 c��v������=v���棩 d�����жϣ�

��ش��������⣺
��1����һ�ܱ������н��з�ӦCH4��g��+H2O��g��?CO��g��+3H2��g����H1=+206.1kJ/mol�����CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ1��ʾ����Ӧ���е�ǰ5min�ڣ�v��H2��=______��10minʱ���ı���������������______��
��2����ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȣ�����ͬ�¶��·�����ӦCH4��g��+CO2��g��?2CO��g��+2H2��g����H2=+247.3kJ/mol����ά�ַ�Ӧ�������¶Ȳ��䣮��֪��������CH4��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��
��3����ӦCO��g��+H2O��g��?CO2��g��+H2��g����H3=-41.2kJ/mol��800��ʱ�Ļ�ѧƽ�ⳣ��K=1.0��ijʱ�̲�ø��¶��µ��ܱ������и����ʵ����ʵ������±���
| CO | H2O | CO2 | H2 |
| 0.5mol | 8.5mol | 2.0mol | 2.0mol |
a��v��������v���棩 b��v��������v���棩 c��v������=v���棩 d�����жϣ�
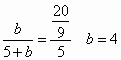


 B(g)+C(g)�������ֲ�ͬ�����½��У�����ʵ�����800���½��У�ʵ�����850���½��У�B��C�ij�ʼŨ�ȶ�Ϊ0����Ӧ��A��Ũ��(mol/L)��ʱ��(min)�ı仯�����ͼ��ʾ���Իش��������⣺
B(g)+C(g)�������ֲ�ͬ�����½��У�����ʵ�����800���½��У�ʵ�����850���½��У�B��C�ij�ʼŨ�ȶ�Ϊ0����Ӧ��A��Ũ��(mol/L)��ʱ��(min)�ı仯�����ͼ��ʾ���Իش��������⣺ 
