��Ŀ����
����Ŀ���ҹ���ѧ�������������о�����ȡ����һϵ�е��ش�ͻ�ƣ���־���ҹ�������̬���������Ѿ���Ϊһ��ǿ����LiZnAs ���о������������ϵ���Ҫǰ�塣
(1)LiZnAs ������Ԫ�صĵ縺�Դ�С�����˳��Ϊ_____��
(2)AsF3 ���ӵĿռ乹��Ϊ_____��As ԭ�ӵ��ӻ��������Ϊ_____��
(3)CO ����������λ���Ĵ��ڣ�ʹ C ԭ���ϵĵ������ܶȽϸ߶�����Ѫ�쵰��ϣ� ���� CO �о綾��1mol[Zn(CN)4]2-�����ں��еĹ��ۼ�����ĿΪ_____����ԭ��Ϊ_____��
(4)���������ﳣ�����缫���ϵĻ��ʡ������ NiO ����Ľṹ�� NaCl �ľ���ṹ��ͬ��Ϊ��ø��õĵ���������������� NiO �����ڿ����м��ȣ�ʹ���� Ni2+�������� Ni3+�� ÿ�������� O2-����Ŀ��λ�þ�δ�����仯�������ӵ�λ����Ȼû�䣬������Ŀ���٣���ɾ����ڲ��������ӿ�λ����ͼ��ʾ������ѧʽΪ NiO ��ij�������ᄃ�壬�����ӵ�ƽ����λ��Ϊ__________�������ӵ�ƽ����λ���봿��� NiO �������____________����������������С����������������д����������Ԫ�ػ��ϼ۵ĸþ���Ļ�ѧʽ________ʾ����Fe3O4 д�� Fe2+Fe23+O4����

(5)���еľ�����ɿ�����ijЩ����һ���ķ�ʽ�ѻ��������ijЩ������������� �����γɵĿ�϶�С��������������ܶѻ��ľ����д����������͵Ŀ�϶���������϶���������϶��������ͼ��ʾ������ LiZnAs ���������У�Zn ������������ʽ�ѻ���Li �� As �ֱ������ Zn ԭ��Χ�ɵİ������϶���������϶�У��� a=0��0.5 �� 1 ���������� Zn �� Li ����ͼ��ʾ�ֲ���������ͼ As ԭ�����ڵĽ������������������� As ԭ�ӵ�λ�ã�______________��˵�� a=__________��


���𰸡�Li��Zn��As ���� sp3 16NA C 6 ��С Ni2+3Ni3+4O9  ��
�� 0.25�� �� 0.75��
0.25�� �� 0.75��
��������
��1����ǻ��ý�����п�ǽϻ��õĽ��������Ƿǽ�����ʧ���������������õ���������ǿ���縺����ǿ��LiZnAs ������Ԫ�صĵ縺�Դ�С�����˳��ΪLi��Zn��As��
�ʴ�Ϊ��Li��Zn��As
��2��AsF3 ���ӣ��۲���Ӷ�=3+![]() =4����һ�Թµ��Ӷԣ��ռ乹��Ϊ������As ԭ�ӵ��ӻ��������Ϊsp3��
=4����һ�Թµ��Ӷԣ��ռ乹��Ϊ������As ԭ�ӵ��ӻ��������Ϊsp3��
�ʴ�Ϊ��������sp3��
��3��CO ����������λ���Ĵ��ڣ�ʹ C ԭ���ϵĵ������ܶȽϸ߶�����Ѫ�쵰��ϣ� ���� CO �о綾��1mol[Zn(CN)4]2-�����ڹ��ۼ���C��N֮����������Zn��C֮������λ�������еĹ��ۼ�����ĿΪ��3��4+4��NA= 16NA�� C ԭ���ϵĵ������ܶȽϸߣ���ԭ��ΪC��
�ʴ�Ϊ�� 16NA��C��
��4������� NiO ����Ľṹ�� NaCl �ľ���ṹ��ͬ�������ӵ����¡�ǰ��������6�������ӣ������ӵ�ƽ����λ��Ϊ6������ Ni2+�������� Ni3+�������Ӽ��٣������ӵ�ƽ����λ���봿��� NiO ������ȼ�С��
���ݻ��ϼ۴����͵���0����Ͼ����ṹ����������Ԫ�ػ��ϼ۵ĸþ���Ļ�ѧʽNi2+3Ni3+4O9����
�ʴ�Ϊ��6����С��Ni2+3Ni3+4O9��
��5������� a=0��0.5 �� 1 ���������� Zn �� Li ����ͼ��ʾ�ֲ��� ���� LiZnAs ���������У�Zn ������������ʽ�ѻ���Li ����� Zn ԭ��Χ�ɵİ������϶���� As ����� Zn ԭ��Χ�ɵ��������϶�У� As ԭ�����ڵĽ��漴 a=0.25�� �� 0.75���Ľ����������������� As ԭ�ӵ�λ�ã���ͼ��
���� LiZnAs ���������У�Zn ������������ʽ�ѻ���Li ����� Zn ԭ��Χ�ɵİ������϶���� As ����� Zn ԭ��Χ�ɵ��������϶�У� As ԭ�����ڵĽ��漴 a=0.25�� �� 0.75���Ľ����������������� As ԭ�ӵ�λ�ã���ͼ�� ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��0.25�� �� 0.75����
��0.25�� �� 0.75����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������ԭ�ζ�ԭ��ͬ�к͵ζ�ԭ�����ƣ�Ϊ�˲ⶨijδ֪Ũ�ȵ�NaHSO3��Һ��Ũ�ȣ�����0.2000mol/L������KMnO4��Һ���еζ����ش��������⣺
(1) ��ƽ���ӷ���ʽ��______MnO4-+HSO3-+H+=Mn2++SO42-+H2O��
(2)��KMnO4���еζ�ʱ��KMnO4��ҺӦװ��_______________�У��жϵζ��յ��������_______��
(3)���в����ᵼ�²ⶨ���ƫ�ߵ���_________��
A. ʢװδ֪ŨҺ����ƿ������ˮϴ����δ�ô���Һ��ϴ
B. δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
C. �۲����ʱ���ζ�ǰ���ӣ��ζ�����
D. �ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
(4) �����±��ⶨ��ʵ�����ݣ�����KMnO4��Һ�����ƽ��ֵΪ___mL��NaHSO3��Һ�����ʵ���Ũ��Ϊ___mol/L�����ݾ�ȷ��0.1����
������ | ����NaHSO3��Һ�����/ mL | KMnO4��Һ���/ mL |
1 | 20.00 | 15.98 |
2 | 20.00 | 17.00 |
3 | 20.00 | 16.02 |
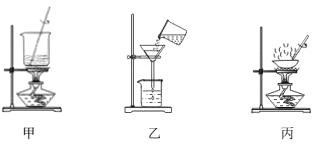
����Ŀ������ʵ��װ���ܴﵽʵ��Ŀ�ĵ��ǣ� ��
|
|
|
|
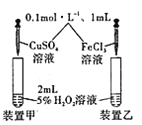
A.�ⶨһ��ʱ�������� H2�ķ�Ӧ���� | B.���Խ����к��ȵIJⶨʵ�� | C.�Ƚ��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� | D.�Ƚ�Cu2+��Fe3+�� H2O2�ֽ����ʵ�Ӱ�� |
A.AB.BC.CD.D