��Ŀ����
����Ŀ�������仯����������������Ӧ����㷺�����������ͺ����²��ϵ�Ӧ�ñ��ܹ�ע���ɴ˲�����![]() �ȷ������������ش�
�ȷ������������ش�

��1����![]() Ӧ�����������ȼ�ϼ״������ܻ�������ЧӦ��Ӱ�죬����Ϊ��Դ���Ʊ������µ���������ϳɷ�ӦΪ
Ӧ�����������ȼ�ϼ״������ܻ�������ЧӦ��Ӱ�죬����Ϊ��Դ���Ʊ������µ���������ϳɷ�ӦΪ![]() ����ͼΪ
����ͼΪ![]() ƽ��ת���ʺ��¶ȡ�ѹǿ�Ĺ�ϵ������ѹǿ�ֱ�Ϊ
ƽ��ת���ʺ��¶ȡ�ѹǿ�Ĺ�ϵ������ѹǿ�ֱ�Ϊ![]() ����ͼ��֪���÷�ӦΪ_______��Ӧ����������������������������
����ͼ��֪���÷�ӦΪ_______��Ӧ����������������������������![]() �ij�ʼŨ��Ϊ
�ij�ʼŨ��Ϊ![]() ������
������![]() ʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��
ʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��![]() _________����ʽ���ɣ�����4.0Mpaʱ��СͶ�ϱ�
_________����ʽ���ɣ�����4.0Mpaʱ��СͶ�ϱ�![]() ����
����![]() ��ƽ��ת�������߿���λ��II�ߵ�_________�������Ϸ��������·�������
��ƽ��ת�������߿���λ��II�ߵ�_________�������Ϸ��������·�������
��2��![]() ʱ����ij�����ܱ������м���һ������
ʱ����ij�����ܱ������м���һ������![]() ��
��![]() ��������Ӧ
��������Ӧ![]() ����Ӧ�ﵽƽ�����
����Ӧ�ﵽƽ�����![]() ʱ�̣��ı�ij������
ʱ�̣��ı�ij������![]() ��ʱ�䣨t���ı仯��ϵ��ͼ1��ʾ����
��ʱ�䣨t���ı仯��ϵ��ͼ1��ʾ����![]() ʱ�̸ı������������______����д��ĸ����
ʱ�̸ı������������______����д��ĸ����
a �����¶Ȳ��䣬ѹ������ b ����������䣬�����¶�
c ����������䣬������̼�� d ����������䣬����![]() Ũ��
Ũ��


��3����һ���¶��£���ij����ɱ�ĺ�ѹ�ܱ�������p�ܣ�����1molCO2��������̼��������Ӧ![]() ��ƽ��ʱ��ϵ����������������¶ȵĹ�ϵ��ͼ2��ʾ����650��ʱ���÷�Ӧ��ƽ������յ�������___________KJ����
��ƽ��ʱ��ϵ����������������¶ȵĹ�ϵ��ͼ2��ʾ����650��ʱ���÷�Ӧ��ƽ������յ�������___________KJ����![]() �Ļ�����壬ƽ��_______________���������������������������������ƶ���
�Ļ�����壬ƽ��_______________���������������������������������ƶ���
��4����֪25��ʱ��![]() �����¶�������ʵ����������100mL 5 molL1FeCl3��Һ��Ϊʹ���ƹ����в����ֻ���������������Ҫ����2 molL1������___________mL�����Լ��������������
�����¶�������ʵ����������100mL 5 molL1FeCl3��Һ��Ϊʹ���ƹ����в����ֻ���������������Ҫ����2 molL1������___________mL�����Լ��������������
���𰸡����� ![]() �Ϸ� d 43 ���� 2.5
�Ϸ� d 43 ���� 2.5
��������
����ͨ���¶����ߣ�ת���ʱ仯��ƽ���ƶ����з������ȸ�����ͬ�¶�ʱ�����µ��ϣ���ת�������ж�I��II��III��ѹǿ���ٽ�������ʽ�õ�ƽ�ⳣ������СͶ�ϱ�![]() ���൱��CO2�������䣬����H2�����ʵ���������ת���ʡ�
���൱��CO2�������䣬����H2�����ʵ���������ת���ʡ�
��a. �����¶Ȳ��䣬ѹ��������Ũ�ȱ�����淴Ӧ���ʼӿ죬���ڵ������Ӧ��ƽ�ⲻ�ƶ���b. ����������䣬�����¶ȣ����淴Ӧ����˲ʱ���ӿ죬����ƽ��ʱ������Ӧ���ʼ�С���淴Ӧ�������ӣ�c. ����������䣬������̼�ۣ����ʲ��䣻d. ����������䣬����![]() Ũ�ȣ��淴Ӧ����˲������ƽ��Σ��淴Ӧ���ʼ�С������Ӧ��������
Ũ�ȣ��淴Ӧ����˲������ƽ��Σ��淴Ӧ���ʼ�С������Ӧ��������
�����ȼ���ƽ��ʱCO�����ʵ������ټ���������������T��ʱ������ͼ��ó�![]() �����Խ�����
�����Խ�����![]() �Ļ�������Ϊ���������г���
�Ļ�������Ϊ���������г���![]() ���ٳ���CO����������ƽ���ƶ���
���ٳ���CO����������ƽ���ƶ���
���ȸ����ܶȻ�����������Ũ�Ⱥ�������Ũ�ȣ��ٸ�����Һϡ��ԭ���������������
�ž�ͼ��֪�������¶ȣ�ת�����½���ƽ�������ƶ������������ȷ�Ӧ�������Ƿ��ȷ�Ӧ����Ӧ�������С�ķ�Ӧ�����¶ȣ����µ��ϣ�ת��������˵���Ǽ�ѹ�����IΪ5.0MPa��IIΪ4.0MPa��IIIΪ3.0MPa����![]() �ij�ʼŨ��Ϊ
�ij�ʼŨ��Ϊ![]() ��
�� ������
������![]() ʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��
ʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��![]() ����4.0Mpaʱ��СͶ�ϱ�
����4.0Mpaʱ��СͶ�ϱ�![]() ���൱��CO2�������䣬����H2�����ʵ�������
���൱��CO2�������䣬����H2�����ʵ�������![]() ��ƽ��ת��������������߿���λ��II�ߵ��Ϸ����ʴ�Ϊ�����ȣ�
��ƽ��ת��������������߿���λ��II�ߵ��Ϸ����ʴ�Ϊ�����ȣ�![]() ���Ϸ���
���Ϸ���
��a. �����¶Ȳ��䣬ѹ��������Ũ�ȱ�����淴Ӧ���ʼӿ죬���ڵ������Ӧ��ƽ�ⲻ�ƶ�����a���������⣻b. ����������䣬�����¶ȣ����淴Ӧ����˲ʱ���ӿ죬����ƽ��Σ�����Ӧ������С���淴Ӧ���������ӣ���b���������⣻c. ����������䣬������̼�ۣ����ʲ��䣬��c���������⣻d. ����������䣬����CO2Ũ�ȣ��淴Ӧ����˲�䣬�����ƽ��Σ��淴Ӧ������С������Ӧ����������d�������⣻����������Ϊ��d��
����650��ʱ���÷�Ӧ��ƽ�⣬CO�������ռ40%����CO���ʵ���Ϊ2x������![]() ��x=0.25mol��CO�����ʵ���Ϊ2x=2��0.25mol=0.5 mol�����ݷ���ʽ����2molCO����172.4kJ����������Ӧ����0.5molCO�������յ�������
��x=0.25mol��CO�����ʵ���Ϊ2x=2��0.25mol=0.5 mol�����ݷ���ʽ����2molCO����172.4kJ����������Ӧ����0.5molCO�������յ�������![]() ���ʴ�Ϊ��43��
���ʴ�Ϊ��43��
��T��ʱ���������������Ϊ50%����![]() �����Խ�����
�����Խ�����![]() �Ļ�������Ϊ���������г���
�Ļ�������Ϊ���������г��� ʱ��ƽ�ⲻ�ƶ����ٳ���CO���壬ƽ�������ƶ����ʴ�Ϊ������
ʱ��ƽ�ⲻ�ƶ����ٳ���CO���壬ƽ�������ƶ����ʴ�Ϊ������
����֪25��ʱ��![]() �����¶�������ʵ����������100mL 5 molL1FeCl3��Һ��
�����¶�������ʵ����������100mL 5 molL1FeCl3��Һ�� ![]() ����
����![]() ��
�� =0.05mol/L����
=0.05mol/L����![]() 0.05mol/Lʱ�����ֻ�������0.05mol/L��0.1L=2mol/L��V����������Ҫ����2 molL1������V=0.0025L =2.5mL���ʴ�Ϊ2.5��
0.05mol/Lʱ�����ֻ�������0.05mol/L��0.1L=2mol/L��V����������Ҫ����2 molL1������V=0.0025L =2.5mL���ʴ�Ϊ2.5��

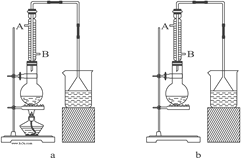
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�����Ŀ������ȩ��ҽҩ��Ⱦ�ϡ����ϵ���ҵ���Ź㷺��Ӧ�á�ʵ����ͨ����ͼ��ʾ�������ɼױ������Ʊ�����ȩ��

�Իش��������⣺
��1��Mn2O3�����ױ��ķ�Ӧ��Ҫ���Ͻ��裬�����������___��
��2���ױ���������õ��Ļ����ͨ���ᾧ�����˽��з��룬�ù������轫�������ȴ����Ŀ��___��
��3��ʵ������У���ѭ��ʹ�õ����ʷֱ�Ϊ___��___��
��4��ʵ���з���ױ��ͱ���ȩ���õIJ���I��___________��
��5��ʵ���з��֣���Ӧʱ�䲻ͬ����ȩ�IJ���Ҳ��ͬ(���ݼ��±�)��
��Ӧʱ��/h | 1 | 2 | 3 | 4 | 5 |
����ȩ����/% | 76.0 | 87.5 | 83.6 | 72.5 | 64.8 |
���ϱ���ȩ�Ľṹ����������Ӧʱ�����ʱ������ȩ�����½���ԭ��___��