��Ŀ����
4����̼���ʾ��������������ʣ�2010��ŵ��������ѧ����ָ�����̼����һ���������ף�������֪�������--ʯīϩ��
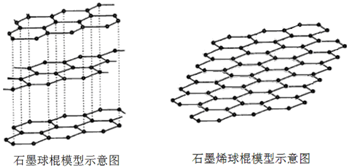
��1������˵���У���ȷ����BD��
A����̬ʱ��̼�ĸ��ֵ��ʵľ���������ͬ
B��ʯīϩֻ���зǼ��Լ�
C����ʯī�����ʯīϩ��Ҫ�ƻ���ѧ��
D��ʯīϩ���е�����
��2����ѧ����������ǻ�ô���ʯīϩ����Ч����֮һ������Ϊ��ͭ�Ȼ�Ͻ𣬺�̼Դ��������Ȳ�������Ҵ����е�һ�ֻ�������ϣ�
���Ҵ��Ͷ����ѣ�H3C-O-CH3����ͬ���칹�壬�����ѵķе���Ҵ��ͣ���ߡ��͡�����ԭ�����Ҵ����Ӽ���γ�������������ѷ��Ӽ��������
��ͭ�ĵ�һ�����ܣ�I1��С��п�ĵ�һ�����ܣ���ͭ�ĵڶ������ܣ�I2��ȴ����п�ĵڶ������ܣ�����Ҫԭ����Cuʧȥһ�����ӳ�ΪCu+��������Ų�Ϊ[Ar]3d10������ȫ�����������ϵͣ��ṹ�ȶ�������Cu��2��������Խϴ�
���� ��1��A��̼���ֵ��ʵľ������Ͳ�ͬ��
B��ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ���
C��ʯī�в�������ڷ��Ӽ���������
D��ʯīϩ����ʯī�����ԣ�
��2�����Ҵ����Ӽ����������Էе�ߣ�
��Cuʧȥһ�����ӳ�ΪCu+��������Ų�Ϊ[Ar]3d10������ȫ�����������ϵͣ�
��� �⣺��1��A��̼���ֵ��ʵľ���������ͬ������ʯ����ԭ�Ӿ��壬����ϩ���ڷ��Ӿ��壬��A����
B��ʯīϩ��̼ԭ�Ӽ京�зǼ��Լ�����B��ȷ��
C��ʯī�в�������ڷ��Ӽ������������Դ�ʯī����õ�ʯīϩ��Ҫ�ƻ����Ӽ�����������C����
D��ʯīϩ�к��������ƶ��ĵ��ӣ����Ծ��е����ԣ���D��ȷ��
��ѡB��D��
��2�����Ҵ����Ӽ����������Էе�ߣ����Զ����ѵķе���Ҵ��ͣ��ʴ�Ϊ���ͣ��Ҵ����Ӽ���γ�������������ѷ��Ӽ��������
��Cuʧȥһ�����ӳ�ΪCu+��������Ų�Ϊ[Ar]3d10������ȫ�����������ϵͣ��ʴ�Ϊ��Cuʧȥһ�����ӳ�ΪCu+��������Ų�Ϊ[Ar]3d10������ȫ�����������ϵͣ��ṹ�ȶ�������Cu��2��������Խϴ�
���� ���⿼����ۺϣ��漰����������ܴ�С���жϵ�֪ʶ�㣬��ȷ�����ܴ�С��ԭ�ӽṹ�Ĺ�ϵ�ǽⱾ��ؼ����Ѷ��еȣ�

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�| A�� | ͬ����Ԫ�غ������������˵���������Ӷ����� | |
| B�� | ��������Ų���ͬ������ѧ����Ҳ��ͬ | |
| C�� | Cl?��S2?��Ca2+��K+�뾶��С | |
| D�� | 35Cl2��37Cl2�õ���������ͬ |
��SiO2��Na2SiO3
��Al��OH��3-��Al2O3
��SiO2��H2SiO3
��Al2O3-��Al��OH��3
��Na2O2��NaCl
��Al-��AlO2-��
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �ݢ� |
| A�� | �����ƽ��Ħ������Ϊ24 g/mol | |
| B�� | ��������ͨ��Ũ���������պ�ָ�����101 kPa��120�棩����ѹǿ��Ϊԭ����$\frac{1}{3}$ | |
| C�� | ��Ӧ�����ĵ�����Ϊ56 g | |
| D�� | ��������ͨ����ʯ�ң����ȫ�����գ���ͨ��Ũ���ᣬ���ܱ���ȫ���� |
| A�� | 10g H${\;}_{2}^{18}$O��������������Ϊ4NA | |
| B�� | 1mol�������к��е�̼̼˫����Ϊ3NA | |
| C�� | ��״���£�22.4LCl2��ȫ��Ӧ��ת�Ƶĵ�����һ����2NA | |
| D�� | �����£�1L0.1mol/L Na2CO3��Һ�е���������������0.1NA |

 ��
��
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$
 ���������֣�
���������֣�