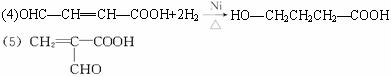��Ŀ����
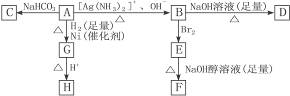
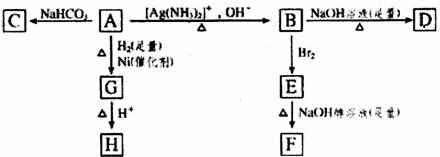
����ͼʾ��գ�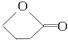
H�ǻ�״������C4H6O2��F��̼ԭ����һ��ֱ���ϡ�
(1)������A���еĹ�������__________��
(2)B��������������Br2��Ӧ�õ�E��E���������������ƴ���Һ������ת��ΪF����Eת��ΪFʱ�������ַ�Ӧ���䷴Ӧ���ͷֱ���__________��
(3)D�Ľṹ��ʽ��_____________________________________________��
(4)1 mol A��2 mol H2��Ӧ����1 mol E���䷴Ӧ����ʽ��__________________________��
(5)��A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ��____________________________��
������A����������Һ��Ӧ��˵��A���С�CHO��������NaHCO3��Ӧ����A�л��С�COOH����A��H2��Ӧ����Gʱ����CHOת��Ϊ��CH2OH��������С�COOH�����С�OH�������������£�����������Ӧ�����ɻ�������C4H6O2������ΪF��̼ԭ����һ��ֱ����(˵��F�д���C��C)����4��Cԭ����֧���ṹ������HΪ![]() ��GΪCH2OHCH2CH2CH2COOH������(4)��1 mol A��2 mol H2��Ӧ�����С�CHO��1 mol H2����˵��A�л���һ��C=C�����AΪOHC��CH=CHCOOH��BΪHOOC��CH=CH��COOH��DΪNaOOC��CH=CH��COONa��CΪOHC��CH=CH��COONa�ڴ�����Һ�У�������ȥ���кͷ�Ӧ�����ɵ�FΪNaOO��C��C��COONa������Ϊ��CHO�롪COOH������̼�����ˣ�����CHO(��COOH)����Ϊȡ�����ţ����Ƶ�C=C����һ��̼ԭ���ϣ�����A��ͬ���칹��(������ͬ������)Ϊ
��GΪCH2OHCH2CH2CH2COOH������(4)��1 mol A��2 mol H2��Ӧ�����С�CHO��1 mol H2����˵��A�л���һ��C=C�����AΪOHC��CH=CHCOOH��BΪHOOC��CH=CH��COOH��DΪNaOOC��CH=CH��COONa��CΪOHC��CH=CH��COONa�ڴ�����Һ�У�������ȥ���кͷ�Ӧ�����ɵ�FΪNaOO��C��C��COONa������Ϊ��CHO�롪COOH������̼�����ˣ�����CHO(��COOH)����Ϊȡ�����ţ����Ƶ�C=C����һ��̼ԭ���ϣ�����A��ͬ���칹��(������ͬ������)Ϊ![]() ��
��
�𰸣�(1)ȩ�����Ȼ���̼̼˫�� (2)��ȥ��Ӧ���кͷ�Ӧ
(3)NaOOC��CH=CH��COONa