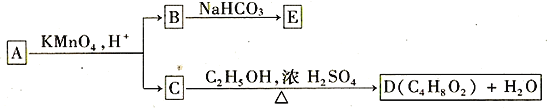
��Ŀ����
����Ŀ����̼��⼼��������ԭ���ԭ������������ˮ��һ�ֹ��գ�װ����ͼ��ʾ�����϶˿��ڹرգ��ɵõ�ǿ��ԭ�Ե�H��(��ԭ��);���϶˿��ڴ�������������ɵõ�ǿ�����Ե���OH���ǻ����ɻ�)������˵���������
A. �����Ƿ��������������ĵ缫��Ӧʽ��ΪFe-2e��===Fe2+
B. ���������ʱ�������ĵ缫��ӦʽΪH++e��===H��
C. �������ʱ��ÿ����1mol��OH��2 mol���ӷ���ת��
D. �������в���(H2C2O4)����ˮʱ���϶˿���Ӧ���������
���𰸡�C
��������
A�������Ƿ�������ʱ������Ϊ��������ʧȥ���������������ӣ��缫��ӦΪ��Fe-2e-=Fe2+��A����ȷ��
B�����������ʱ�������Ͽɵõ�ǿ��ԭ�Ե���ԭ�ӣ��缫��ӦʽΪH++e-=H����B����ȷ��
C�����϶˿ڴ�������������ɵõ�ǿ�����Ե��ǻ����ɻ����缫��ӦΪ2H++2e-+O2=2OH-������ÿ����1mol�ǻ����ɻ���1mol���ӷ���ת�ƣ�C�����
D����ȥ������Ҫ�����Ե����ʣ��϶˿ڴ������ǻ����ɻ���D����ȷ��
���Դ�ѡ��C�

����Ŀ��������Ҫ�Ļ�������ԭ�ϣ������Ʊ������ᣨHNO2�������������ᣨH2N2O2��������[CO��NH2��2]�ȶ��ֺ����Ļ�����Ʒ��
��1��ˮ�ܷ�����ż����2H2O![]() H3O++OH����Һ����ˮ���ѵ��룬��д��Һ������ż���뷽��ʽ_______��
H3O++OH����Һ����ˮ���ѵ��룬��д��Һ������ż���뷽��ʽ_______��
��2��25��ʱ�������������������ĵ��볣�����±���ʾ��
��ѧʽ | HNO2 | H2N2O2 |
���볣�� | Ka=5.1��10��4 | Ka1=6.17��10��8��Ka2=2.88��10��12 |
�����ʵ���Ũ����ͬ��NaNO2��NaHN2O2��Һ��pH��NaNO2��_____pH��NaHN2O2�������������������������
��25��ʱNaHN2O2��Һ�д���ˮ��ƽ�⣬��ˮ�ⳣ��Kh=_______��������λ��Ч���֣���
��0.lmol/LNa2N2O2��Һ������Ũ���ɴ�С��˳��Ϊ____________��
��3����NH3��CO2Ϊԭ�Ͽ��Ժϳ�����[CO��NH2��2]���漰�Ļ�ѧ��Ӧ���£�
��ӦI��2NH3��g��+CO2��g��![]() NH2CO2NH4��s�� ��H1=-159.5kJmol-1��
NH2CO2NH4��s�� ��H1=-159.5kJmol-1��
��ӦII��NH2CO2NH4��s��![]() CO��NH2��2��s��+H2O��g�� ��H2=+116.5kJmol-1��
CO��NH2��2��s��+H2O��g�� ��H2=+116.5kJmol-1��
��ӦIII��H2O��l���TH2O��g�� ��H3=+44.0kJmol-1��
��Ӧ����NH3��CO2�ϳ�����ͬʱ����Һ̬ˮ���Ȼ�ѧ����ʽΪ____________��
��4��T1��ʱ�����ݻ�Ϊ2L�ĺ����ܱ������г���n��NH3��:n��CO2��=2:l��ԭ������ʹ֮������Ӧ������Ӧ������õ����ص�����Ϊ30g�������ڵ�ѹǿp��ʱ��t�ı仯��ͼ1��ʾ��
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K��ֵΪ___________��
��ͼ2������ȷ��Ӧƽ�ⳣ��K���¶ȱ仯��ϵ������Ϊ__________������ĸ��ţ���

��5�������ױ�����������̼����������ˮ��Һ���ö��Ե缫����Ƶ���ϩ����ԭ����ͼ3��ʾ����b�缫�ϵĵ缫��ӦʽΪ______________��
����Ŀ����1�����Ŵ�����Ⱦ���������أ�����������ʮ�������ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ��Լ���10%��������̼(CO2)���ŷ���ҲҪ������١�
����֪��Ӧ��NO2(g)+SO2(g)![]() SO3(g)+NO(g)��һ�������£���NO2��SO2�������1��2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬����____(����ĸ)��
SO3(g)+NO(g)��һ�������£���NO2��SO2�������1��2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬����____(����ĸ)��
A����ϵѹǿ���ֲ���
B�����������ɫ���ֲ���
C����������ƽ����Է����������ٱ仯
D��ÿ����1molSO3��ͬʱ����1 molNO2
��CO2��ת�����л��� CH3OHʵ��̼ѭ������1L���ܱ������У�����lmolCO2�� 3molH2��һ�������·�Ӧ��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)=_______
CH3OH(g)+H2O(g)���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)=_______

�۹�ҵ�ϣ�CH3OH Ҳ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g)![]() CH3OH(g)��ƽ�ⳣ��������˵����ȷ����_____����˫ѡ��
CH3OH(g)��ƽ�ⳣ��������˵����ȷ����_____����˫ѡ��
�¶�/�� | 0 | 100 | 200 | 300 | 400 |
ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�ڵ����²����Է����У������¿��Է�����
C����T��ʱ��1L�ܱ�������Ͷ��0.1molCO�� 0.2molH2���ﵽƽ��ʱ��COת����Ϊ 50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ(5MPa)��250�棬����Ϊ�������£�ԭ����ת�������
��2����NH3��CO2Ϊԭ�Ͽ��Ժϳ�����[CO(NH2)2]���漰�Ļ�ѧ��Ӧ���£�
��ӦI��2NH3(g)+CO2(g)![]() NH2CO2NH4(s) ��H1=159.5 kJ��mol1��
NH2CO2NH4(s) ��H1=159.5 kJ��mol1��
��ӦII��NH2CO2NH4(s)![]() CO(NH2)2(s)+H2O(g) ��H2=+116.5 kJ��mol1��
CO(NH2)2(s)+H2O(g) ��H2=+116.5 kJ��mol1��
![]() H2O(g) ��H3=+44.0 kJ��mol1��
H2O(g) ��H3=+44.0 kJ��mol1��
��ӦIV��NH3��CO2�ϳ�����ͬʱ����Һ̬ˮ���Ȼ�ѧ����ʽΪ_________��
��3��T1��ʱ�����ݻ�Ϊ2L�ĺ����ܱ������г���n(NH3)��n(CO2)=2��1��ԭ������ʹ֮������ӦIV����Ӧ������õ����ص�����Ϊ30g�������ڵ�ѹǿ(p)��ʱ��(t)�ı仯��ͼ1��ʾ����M(����)=60g/mol��
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K��ֵΪ______��


��ͼ2������ȷ��ӳƽ�ⳣ��K���¶ȱ仯��ϵ������Ϊ____�������߱����ĸ��