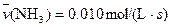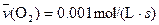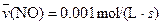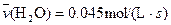��Ŀ����
���ܱ������н��е����·�Ӧ��2SO2��g��+O2(g)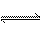 2SO3(g) ��SO2����ʼŨ����0.4 mol��L-1,O2����ʼŨ����1 mol��L-1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬
2SO3(g) ��SO2����ʼŨ����0.4 mol��L-1,O2����ʼŨ����1 mol��L-1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬
��1����Ӧ��ƽ�ⳣ����
��2������ƽ��ʱ��Ӧ������ѹǿ����1����ƽ�⽫����ƶ���
��3����ƽ��ʱ��Ӧ������ѹǿ��С1����ƽ�⽫����ƶ���
��4��ƽ��ʱ����������䣬��ƽ���������г���ϡ������Ar,ʹ��ϵ��ѹ��Ϊԭ����3����ƽ���ֽ�����ƶ���
 2SO3(g) ��SO2����ʼŨ����0.4 mol��L-1,O2����ʼŨ����1 mol��L-1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬
2SO3(g) ��SO2����ʼŨ����0.4 mol��L-1,O2����ʼŨ����1 mol��L-1����SO2��ת����Ϊ80%ʱ����Ӧ�ﵽƽ��״̬��1����Ӧ��ƽ�ⳣ����
��2������ƽ��ʱ��Ӧ������ѹǿ����1����ƽ�⽫����ƶ���
��3����ƽ��ʱ��Ӧ������ѹǿ��С1����ƽ�⽫����ƶ���
��4��ƽ��ʱ����������䣬��ƽ���������г���ϡ������Ar,ʹ��ϵ��ѹ��Ϊԭ����3����ƽ���ֽ�����ƶ���
(1)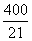 (2)������Ӧ�����ƶ� (3)���淴Ӧ�����ƶ� (4)���ƶ�
(2)������Ӧ�����ƶ� (3)���淴Ӧ�����ƶ� (4)���ƶ�
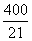 (2)������Ӧ�����ƶ� (3)���淴Ӧ�����ƶ� (4)���ƶ�
(2)������Ӧ�����ƶ� (3)���淴Ӧ�����ƶ� (4)���ƶ�2SO2��g�� + O2��g��
 2SO3��g��
2SO3��g��2 1 2
��ʼʱ 0.4 1 0
ƽ��ʱ 0.4��1-80%�� 1-1/2��0.4 0.4��80%
=0.08 ��80% =0.84 =0.32
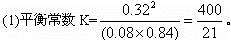

��Qc��K������ƽ��������Ӧ�����ƶ���
(3)ѹǿ��С1��������ֵ�Ũ��Ҳ��С1����
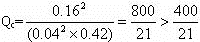
��Qc��K������ƽ�����淴Ӧ�����ƶ���
(4)����������䣬����ϡ������Ar��������ѹ�ı䣬����Ӧ��������ֵ�Ũ�ȱ��ֲ��䡣
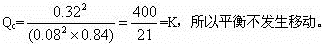

��ϰ��ϵ�д�
�����Ŀ
 2HI(g)��H��0,���ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��
2HI(g)��H��0,���ﵽƽ�⡣HI���������w(HI)��ʱ��t�仯��ͼ����(��)��ʾ��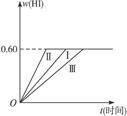

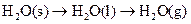
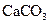 �T�T
�T�T 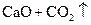
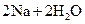 �T�T
�T�T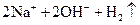
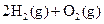 �T�T
�T�T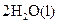
 pC(g)+qD(g),������C�����������ѹǿp1��p2��ʱ��t1��t2�Ĺ�ϵ����ͼ��ʾ�������й�ϵ��ȷ����( )
pC(g)+qD(g),������C�����������ѹǿp1��p2��ʱ��t1��t2�Ĺ�ϵ����ͼ��ʾ�������й�ϵ��ȷ����( )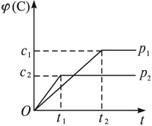
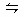 b B��g��+c C��g�������¶Ȳ���������£��ٳ���һ������A ���ʣ����´ﵽƽ��ʱ�������ж�����ȷ���ǣ� ��
b B��g��+c C��g�������¶Ȳ���������£��ٳ���һ������A ���ʣ����´ﵽƽ��ʱ�������ж�����ȷ���ǣ� �� bB(g)�ﵽƽ������¶Ȳ��䣬�������������1�������ﵽ��ƽ��ʱ��8��Ũ����ԭ����60��������˵����ȷ����
bB(g)�ﵽƽ������¶Ȳ��䣬�������������1�������ﵽ��ƽ��ʱ��8��Ũ����ԭ����60��������˵����ȷ����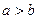
 4NO(g)+6H2O(g)��10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������
4NO(g)+6H2O(g)��10L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45mol����˷�Ӧ��ƽ������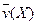 ����Ӧ����������ʻ������������ʣ��ɱ�ʾΪ�� ��
����Ӧ����������ʻ������������ʣ��ɱ�ʾΪ�� ��