��Ŀ����
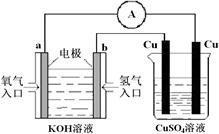
��֪Ǧ���صĹ���ԭ��ΪPb��PbO2��2H2SO4 2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ� Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش� �������⡣
2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ� Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش� �������⡣

(1)A��Ǧ���ص� ����Ǧ����������ӦʽΪ ���ŵ�����е��Һ���ܶ� (���С�����������䡱)��
(2)Ag�缫�ĵ缫��Ӧʽ�� ���õ缫�ĵ缫���ﹲ g��
(3)Cu�缫�ĵ缫��Ӧʽ�� ��CuSO4��Һ��Ũ�� (���С�����������䡱)
(4)��ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ ��

a����U�ι��в�������������
b����U�ι������������ļ�����
c����U�������������������
 2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ� Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش� �������⡣
2PbSO4��2H2O��������ͼװ�ý��е��(���Һ����)����õ� Ǧ������ת��0.4 mol����ʱ���缫����������11.2 g����ش� �������⡣
(1)A��Ǧ���ص� ����Ǧ����������ӦʽΪ ���ŵ�����е��Һ���ܶ� (���С�����������䡱)��
(2)Ag�缫�ĵ缫��Ӧʽ�� ���õ缫�ĵ缫���ﹲ g��
(3)Cu�缫�ĵ缫��Ӧʽ�� ��CuSO4��Һ��Ũ�� (���С�����������䡱)
(4)��ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ ��

a����U�ι��в�������������
b����U�ι������������ļ�����
c����U�������������������
(1)����PbO2��4H����SO42-��2e��=PbSO4��2H2O����С
(2)2H����2e��=H2����0.4
(3)Cu��2e��=Cu2��������
(4)b
(2)2H����2e��=H2����0.4
(3)Cu��2e��=Cu2��������
(4)b
�����ڵ����������缫�����ļ��ٿ��ж�A�ǵ�Դ�ĸ�����B�ǵ�Դ�����������ʱAg�����������缫��ӦʽΪ2H����2e��=H2����Fe���������缫��ӦʽΪFe��2e��=Fe2�������U�ι����ܷ�ӦʽΪFe��2H��=Fe2����H2�����Ҳ�U�ι��൱�ڵ��װ�ã�Zn�缫���������缫��ӦʽΪCu2����2e��=Cu��ͭ�缫���������缫��ӦʽΪCu��2e��=Cu2������ƹ�����CuSO4��Һ��Ũ�ȱ��ֲ��䣬�������������ɵô𰸡�

��ϰ��ϵ�д�
�����Ŀ

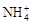 ��2e����Mn2O3��2NH3��H2O��
��2e����Mn2O3��2NH3��H2O��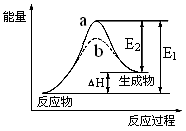
 CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1