��Ŀ����
9���������кܶ������뻯ѧ�Ĺ�ϵ�ܲ��ɷ֣��������н��Ͳ���ѧ���ǣ�������| A�� | ��ˮ��ʯ������Ҫ���ܽ���CO2����ˮ��CaCO3�������������˿�����Ca��HCO3��2��Ե�� | |
| B�� | ����ʢ��NaOH��Һ���Լ�ƿ���״�����ΪNaOH��ƿ�е�CO2��Ӧ����ƿ����������γɸ�ѹ��Ե�� | |
| C�� | �ϸ�ؽ���ʵ����ʹ�á�ͨ���������Ⱦ�Dz������εģ���Ϊʵ��������к�����û�еõ�ת�������� | |
| D�� | �����ʺ硱�롰������¥��������Ȼ��Ĺ�ѧ����Ҳ�뽺��֪ʶ�й� |
���� A��̼����������̼��ˮ��Һ��Ӧ���ɿ����Ե�̼����ƣ�
B���������������������Һ��Ӧ���ɵĹ�������ճ�����ʣ�
C��ͨ�������û���к������ת�������գ�ֻ������ļ��ſգ�
D��������һ�ֽ��壮
��� �⣺A��̼����������̼��ˮ��Һ��Ӧ���ɿ����Ե�̼����ƣ������ܽ���CO2����ˮ�ܰ�ʯͷ������A��ȷ��
B�������к��ж������裬�������������������Һ��Ӧ����ճ�ԵĹ����ƣ��Ӷ�ʹ�������Ͳ���ƿճ����һ����Ѵ���B����
C��ͨ�������û���к������ת�������գ�ֻ������ļ��ſգ���Ȼ����Ⱦ����������ͨ�������ʹ����һ�ֲ������εķ���Ⱦ�ֶΣ���C��ȷ��
D������Ҳ��һ�ֽ��壬��������¥���ǹ��ߵķ��䣬�����ʺ硱�ǽ���Ķ����ЧӦ����D��ȷ��
��ѡB��
���� ���⿼�����ʵ����ʣ���ȷ�����ijɷּ��������ǽⱾ��ؼ����ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
20��������Ӧ���л���ѧ�е�һ����Ҫ��Ӧ�����ж�������Ӧ���ⲻ��ȷ���ǣ�������
| A�� | ������Ӧ�ķ�Ӧ��֮һ�϶��Ǵ� | B�� | ������Ӧһ�㲻��Ҫ���� | ||
| C�� | ������Ӧ�����ȵ� | D�� | ������Ӧһ����Ҫ���� |
14����ˮ�DZ������Ȼ��Դ������ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��
��1�����á���������������Ũ��ˮ����Br2�����ô������գ���������Ŀ����ʹBr2�����������������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������0.15mol Br2ʱ��ת�Ƶĵ���Ϊ0.25mol��
��2����ˮ��þ��һ�ι���������ͼ��
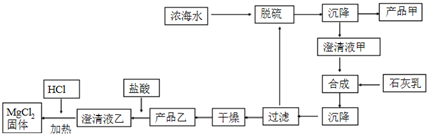
Ũ��ˮ����Ҫ�ɷ����£�
�ٸù��չ����У��������Ҫ�����ӷ���ʽΪCa2++SO42-=CaSO4��������ʯ����ʱ�����������ӷ���ʽ��Mg2++Ca��OH��2=Mg��OH��2��+Ca2+
�ڲ�Ʒ�ҵĻ�ѧʽΪMg��OH��2��1LŨ��ˮ���ɵõ���Ʒ�ҵ�������69.9g��
��1�����á���������������Ũ��ˮ����Br2�����ô������գ���������Ŀ����ʹBr2�����������������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+NaHCO3������0.15mol Br2ʱ��ת�Ƶĵ���Ϊ0.25mol��
��2����ˮ��þ��һ�ι���������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
�ڲ�Ʒ�ҵĻ�ѧʽΪMg��OH��2��1LŨ��ˮ���ɵõ���Ʒ�ҵ�������69.9g��
1��������������ijЩ���⣬�����漰�ڻ�ѧ֪ʶ�����и�����������ȷ���ǣ�������
| A�� | ��Ϻ�ž��˻�����������ȳ���ζ��Ӧ����ˮ��ϴ���������ʱ��������ʳ�� | |
| B�� | ����ϩ������Ʒ�Ƿǻ����Ѻò��ϣ���Ӧ�����ʹ������ϳɲ��� | |
| C�� | ��������ҧ��е���ʹ���̣����Ƿ���ҧ��ʱ������ע�������Ե�ʣ���ʱ����ͿĨϡ��ˮ��̼��������Һ�����Լ�����ʹ | |
| D�� | ҽ�þƾ�������Ƥ����������ԭ�����ڿ���ʹ�����ʷ������� |
 ��Ԫ��Y�����ڱ���λ�ڵڢ�A�壮
��Ԫ��Y�����ڱ���λ�ڵڢ�A�壮

 ��
�� ��
��