��Ŀ����
����ͭ�����׳ơ������������������������������й㷺��Ӧ�á�ij�����о�С���ͬѧ�ô�ͭ�ۣ�����̼�����ʣ�����������Ʊ�������;�������ⶨ�����нᾧˮ�ĺ�������Ƶ��������£�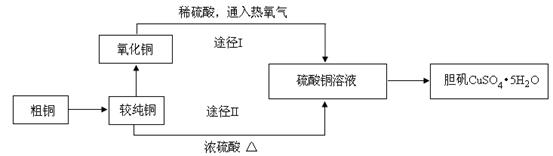
��1�����ϴ�ͭ��ת��Ϊ����ͭʱ��Ӧ�������� �ڽ������գ���д�������ƣ�������ͭ���������֬�������ȼ���Һϴȥ��ԭ���� �������ա���ͭ������õIJ����ǻ�������ͭ������ͭ����������ͭ�Ŀ���ԭ���� ��
a�����չ����в�������ͭ����ԭ b����������ͭ������������
c������ͭ�ڼ��ȹ����зֽ�����ͭ d�����ղ����ͭδ����ȫ����
��2��ͨ��;��Iʵ���ô�������ͭ��ȡ������������е�ʵ����������ǣ����ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ����Ƚ��ɴ�������ͭ��ȡ����������;����;���������Ե������ŵ㣺
���������������������� ���� ��
�� ��
��3���ⶨ����������ᾧˮ�ĺ���ʱ�����ⶨ������������㣬���������ԭ�������___________��
a�����Ⱥ�����δ�������������ȴ
b��������μ��Ⱥ���������ϴ�
c������ǰ����ʱ����δ��ȫ����
d�����ȹ�������������ʧ
��4��������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⣬A���Թ���ʣ���ɫ��ĩ���ô����ǵ�ľ�����뼯��ƿD������ľ���ܸ�ȼ����Ӧǰ���װ�õ�������ͼ�·��ı�����ʾ��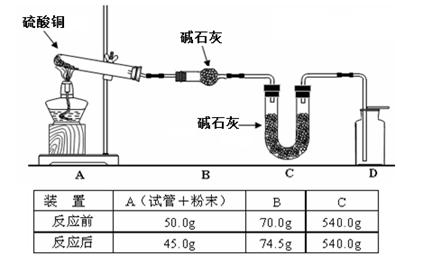
��ͨ�����㣬�ƶϸ�ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ�� ��
��1����������֬�ڼ��������·���ˮ�����ȥ����2�֣���1�֣��� ad��2�֣���1�֣�
��2������Ũ�� ����ϴ�ӣ�2�֣���1�֣����ٲ�������������;��I���������٣���;��I��������Ⱦ���������壨2�֣���1�֣�
��3��cd��2�֣���1�֣�
��4�� ��2�֣���������ȷ1�֣���ƽ1�֣�
��2�֣���������ȷ1�֣���ƽ1�֣�
���������������1����������������¶Ƚϸ�Ӧѡ������������ͭ���������֬�������ȼ���Һϴȥ��ԭ������֬�ڼ��������·���ˮ�����ȥ�������ա���ͭ������õIJ����ǻ�������ͭ������ͭ����������ͭ�Ŀ���ԭ����a�����չ����в�������ͭ����ԭ d�����ղ����ͭδ����ȫ������
��2���������Ҫ��������Ũ��Ȼ����ȴ�ᾧ������ϴ�ӣ��������Ȼ���;���������Ե������ŵ㣺�ٶ��շ�Ӧת�������֪��������������;��I���������٣���;��I��������Ⱦ���������塣
��3���ⶨ�������������˵��ˮ�����϶�������������٣�a�����Ⱥ�����δ�������������ȴ��һ��������b��������μ��Ⱥ���������ϴ�Ҳ���ܻ�ʹˮ��������С�� c������ǰ����ʱ����δ��ȫ�������ˮ����ʹˮ�������࣬��ȷ��d�����ȹ�������������ʧ������ʹʣ�µĹ����������٣��൱��ˮ�������࣬��ȷ��
��4��������ˮ����ͭ��ĩֱ����ȫ�ֽ⣬A���Թ���ʣ���ɫ��ĩ���ô����ǵ�ľ�����뼯��ƿD������ľ���ܸ�ȼ��˵�����������ɡ��ɼ�¼�����ݿɵó�������������������4.5g������������0.5g�����ɹ�������45g�����ݵ����غ㣬����������0.5g��ʧ������(0.5/32)*4mol���õ��ӿ����������˶����������ɶ����������Ϊ��(0.5/32)*2mol������Ϊ2g��������������Ӧ����������������Ϊ2.5g�����ʵ���Ϊ2.5/80mol,�����ʵ���֮�ȣ���������������������=2��2��1������ԭ���غ��֪���ɺ�ɫ����Ϊ����ͭ����ѧ����ʽΪ ��
��
���㣺���⿼����ʵ���ԭ����������������

ij�о���ѧϰС��Ϊ�ⶨNH3�����е�����ԭ�Ӹ����ȣ��������ʵ�����̣�
ʵ��ʱ�������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ��
��ͼA��B��CΪ��С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��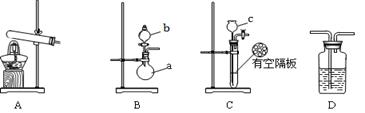
��ش��������⣺
��1��д�����������ƣ�a ��b ��
��2��Ӳ�ʲ������з����ķ�Ӧ����ʽ�� ������Ӧ������Ӳ�ʲ����ܵ������� ��
��3�����ж���ȡ���������õ���װ�ã����±�������Ϊ���е�װ������д��Ӧ��ʵ��ҩƷ��д����ѧʽ����
| װ�� | ʵ��ҩƷ |
| A | |
| B | b�� a�� |
| C | c�� ���壺 |
��4��ʵ��ʱϴ��ƿD�е�Ũ������� �ԣ���С��ʵ���ã�ϴ��ǰװ��D������Ϊm1g��ϴ����װ��D������Ϊm2g�����ɵĵ����ڱ�״���µ����ΪV L�����ݸ�С�����NH3�����е������ԭ�Ӹ����ȵı���ʽ����Ԥ�Ƹý��������ֵ��ȣ� ��
A����ʵ����Χ����ֵ�ӽ�����ֵ B����ֵƫ�� C����ֵƫ��
�±��е�ʵ������ܴﵽʵ��Ŀ�Ļ��ܵó���Ӧ���۵���
| ѡ�� | ʵ������ | ʵ��Ŀ�Ļ�ʵ����� |
| A | ��ʢ��2 mL 0.1 mol/L AgNO3��Һ���Թ��еμ�5��0.1 mol/L NaCl��Һ���а�ɫ�������ɣ��������еμ�5��0.1 mol/L KI��Һ | ˵��һ�ֳ�����ת��Ϊ�ܽ�ȸ�С�ij��� |
| B | ��1 mL 20% ��������Һ�м���3��5��ϡ���ᣬˮԡ����5 min����ȴ���ټ�������Cu(OH)2����Һ������ | ֤�������ܷ���ˮ�ⷴӦ |
| C | ˮԡ����Ũ���ᡢŨ����ͱ��Ļ�����ֱ�������Һ��õ��Ĵֲ�Ʒ | �Ʊ��������� |
| D | ������,�ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ,�ٷֱ����������ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
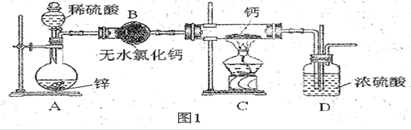
��ͼ��ʾ��һ��ʵ��������װ�ã����ڷ�����������ռ����塣���и�������������������װ�ý���ʵ�����
| A��ͭм��ϡ���� |
| B���������̺�Ũ���� |
| C����Ũ��ˮ����ʯ�ҷ�Ӧ |
| D��̼��ƺ�ϡ���� |



 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C�� MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��