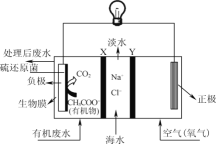
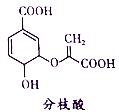
��Ŀ����
����Ŀ���к��ȵIJⶨʵ����ȡ![]() ��NaOH��Һ50mL��
��NaOH��Һ50mL��![]() ������50mL������ͼ��ʾ��װ���У������к��ȵIJⶨʵ�飬�ش��������⣺
������50mL������ͼ��ʾ��װ���У������к��ȵIJⶨʵ�飬�ش��������⣺

(1)��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ______ ��
(2)���ձ����粻��Ӳֽ�壬��õ��к�����ֵ____![]() �ƫ����ƫС������Ӱ�족
�ƫ����ƫС������Ӱ�족![]() ��
��
(3)��![]() ��Һ��
��Һ��![]() ������Һ���ܶȶ���
������Һ���ܶȶ���![]() ���кͺ�������Һ�ı�����
���кͺ�������Һ�ı�����![]() ��ͨ���������ݼ����к���
��ͨ���������ݼ����к���![]() _______
_______![]() �������С�����һλ
�������С�����һλ![]() ��
��
�¶�ʵ����� | ��ʼ�¶� | ��ֹ�¶� | ||
| NaOH | ƽ��ֵ | ||
1 |
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
|
(4)�����![]() ������
������![]() ��Һ���з�Ӧ��������ʵ����ȣ����ų�������____
��Һ���з�Ӧ��������ʵ����ȣ����ų�������____![]() ���ȡ�����ȡ�
���ȡ�����ȡ�![]() �������к���____
�������к���____![]() ���ȡ�����ȡ�
���ȡ�����ȡ�![]() ��
��
(5)����ͬŨ�Ⱥ�����İ�ˮ![]() ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��____��
����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��____��![]() �ƫ����ƫС��������Ӱ�족
�ƫ����ƫС��������Ӱ�족![]() ��
��
���𰸡����β�������� ƫС ![]() ����� ��� ƫС
����� ��� ƫС
��������
��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2�����������ʧ��ʵ���Ӱ�����ش�
��3���ȼ����ÿ��ʵ������ⶨ���¶ȲȻ���������ϴ�����ݣ���������¶Ȳ�ƽ��ֵ������Q=mc��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����
��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
��2�����ձ����粻��Ӳֽ�壬��������ʧ������ʹ����¶Ȳ��С������ʹ���ƫС���ʴ�Ϊ��ƫС��
��3����һ�βⶨ�¶Ȳ�Ϊ����29.5-26.1����=3.4�棬�ڶ��βⶨ���¶Ȳ�Ϊ����32.3-27.2����=5.1�棬�����βⶨ���¶Ȳ�Ϊ����29.2-25.9����=3.3�棬���Ĵβⶨ���¶Ȳ�Ϊ����29.8-26.3����=3.5�棬���еڶ��ε��¶Ȳ����ϴ�Ӧ�����������������¶Ȳ��ƽ��ֵΪ��![]() ��=3.4�棬50 mL 0.55 mol/L����������50 mL 0.25 mol/L������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025 mol����Һ������Ϊ��100 mL��1 g/cm3=100 g���¶ȱ仯��ֵΪ��T=3.4�棬������0.025 molˮ�ų�������Ϊ��Q=mc��T=100g��4.18J/��g�棩��3.4��=1421.2 J����1.4212 kJ������ʵ���õ��к��ȡ�H=-
��=3.4�棬50 mL 0.55 mol/L����������50 mL 0.25 mol/L������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025 mol����Һ������Ϊ��100 mL��1 g/cm3=100 g���¶ȱ仯��ֵΪ��T=3.4�棬������0.025 molˮ�ų�������Ϊ��Q=mc��T=100g��4.18J/��g�棩��3.4��=1421.2 J����1.4212 kJ������ʵ���õ��к��ȡ�H=-![]() =-56.8 kJ/mol���ʴ�Ϊ��-56.8kJ/mol��
=-56.8 kJ/mol���ʴ�Ϊ��-56.8kJ/mol��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��Ⱦ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ��к�����ֵ��ȣ��ʴ�Ϊ������ȣ���ȣ�
��5��һˮ�ϰ�Ϊ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������С��57.3kJ���ʴ�Ϊ��ƫС��

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�����Ŀ������A��D���鷴Ӧ�У���͢����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
ѡ�� | �� | �� |
A | �ѽ���������ϡ������ | �ѽ���������ϡ������ |
B | �Ȼ�����Һ�м���������NaOH��Һ | NaOH��Һ�м����������Ȼ�����Һ |
C | ϡ�����м�������������������Һ | ����������Һ�м���������ϡ���� |
D | ������Na2CO3��Һ��������HCl��Һ�� | ������HCl��Һ��������Na2CO3��Һ�� |
A.AB.BC.CD.D
����Ŀ��������Ϊ����һ����������Ϊ������Ԫ�����������γɵ���������dz��ḻ������������������;�㷺��
��1����̬Sԭ����___��������ͬ�ĵ��ӣ���۵����Ų�ͼΪ___��
��2�������γ�S2O![]() ��SO
��SO![]() �ȶ��ֺ�����������Ʋ�S2O
�ȶ��ֺ�����������Ʋ�S2O![]() �Ŀռ乹��Ϊ___��SO
�Ŀռ乹��Ϊ___��SO![]() ������ԭ���ӻ���ʽΪ___��
������ԭ���ӻ���ʽΪ___��
��3��SCN-��Fe3+�ܷ�����ɫ��Ӧ���÷�Ӧ����������Fe3+�Ĵ��ڡ�
����Ԫ��λ��Ԫ�����ڱ���___����
��SCN-������Ԫ�صĵ縺���ɴ�С��˳��Ϊ___����Ԫ�ط��ű�ʾ����д����SCN-��Ϊ�ȵ�����ķ��ӵķ���ʽ___����дһ�֣���
��Fe(SCN)3�в����ڵĻ�ѧ����___�����ţ���
A.���Ӽ� B.���Լ� C.�Ǽ��Լ� D.��λ�� E.���� F.����
��4������±�������۵����±���ʾ��
±���� | FeF3 | FeCl3 |
�۵�/�� | 1100 | 306 |
���ͱ�������֮���۵�����ԭ��___��
��5��ij�����������������ᄃ����ͼ��ʾ������A��B���ַ�����ɡ�

�ٸ���������Fe2+��Fe3+��O2-���������������Ϊ___��
�ڼ�֪�þ�����ܶ�Ϊdg��cm-3�������ӵ�������ֵΪNA��������aΪ___ nm���ú�d��NA�Ĵ���ʽ��ʾ����