��Ŀ����
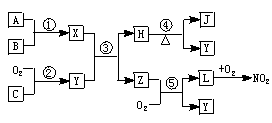
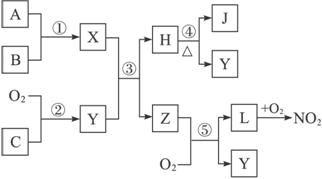
A��B��C�ǵ��ʣ�����A�ǽ������������ʼ��ת����ϵ��ͼ��
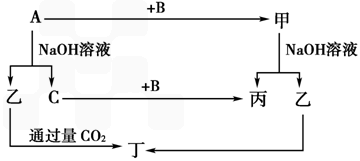
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ �� A________��B________����_______����________��
(2)д�����б仯�Ļ�ѧ����ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ______________________________��
�ڼ���NaOH��Һ��Ӧ�����ӷ���ʽ ____________________________��
(3)��һ������A�� �뵽NaOH��Һ�У�������C�ڱ�״���µ����Ϊ3.36 L�������ĵ�A�����ʵ���Ϊ________��ת�Ƶ��ӵ����ʵ���Ϊ________��
(1)д���������ʵĻ�ѧʽ �� A________��B________����_______����________��
(2)д�����б仯�Ļ�ѧ����ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ______________________________��
�ڼ���NaOH��Һ��Ӧ�����ӷ���ʽ ____________________________��
(3)��һ������A�� �뵽NaOH��Һ�У�������C�ڱ�״���µ����Ϊ3.36 L�������ĵ�A�����ʵ���Ϊ________��ת�Ƶ��ӵ����ʵ���Ϊ________��
(1)Al��O2��NaAlO2��Al(OH)3
(2)��2Al��2NaOH��2H2O===2NaAlO2��3H2������Al2O3��2OH��===2AlO2-��H2O
(3)0.1 mol��0.3 mol
(2)��2Al��2NaOH��2H2O===2NaAlO2��3H2������Al2O3��2OH��===2AlO2-��H2O
(3)0.1 mol��0.3 mol

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ

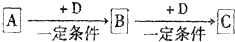 ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ������������ͼ��ʾת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ������������ͼ��ʾת����ϵ��