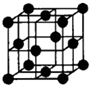
��Ŀ����
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ
��2��B���⻯��ķ��ӿռ乹����
��3��д��������AC2�ĵ���ʽ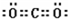
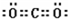 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ
��4��E�ĺ�������Ų�ʽ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ
C��O��N
C��O��N
����2��B���⻯��ķ��ӿռ乹����
������
������
��������ԭ�Ӳ�ȡsp3
sp3
�ӻ�����3��д��������AC2�ĵ���ʽ
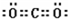
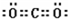
N2O
N2O
����4��E�ĺ�������Ų�ʽ��
1s22s22p63s23p63d54s1
1s22s22p63s23p63d54s1
��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3
[Cr��NH3��4��H2O��2]Cl3
����5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O
4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O
��������A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
����⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�������Ӧ���������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪCrԪ�أ�
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��2��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ�������ͣ�sp3��
��3��������AC2��CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ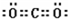 ��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
�ʴ�Ϊ��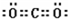 ��N2O��
��N2O��
��4��EΪCrԪ�أ�ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���������Ų�ʽ�� 1s22s22p63s23p63d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ����ΪHNO3��D�ĵ���ΪMg��HNO3ϡ��Һ��Mg��Ӧʱ��NԪ�ر���ԭ����ͼۣ�������NH4NO3��Mg������ΪMg��NO3��2����NH4NO3��Mg��NO3��2�Ļ�ѧ�������ֱ�Ϊx��y������ݵ���ת���غ���[5-��-3��]��x=2y������x��y=4��1���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�EΪCrԪ�أ�
��1��AΪ̼Ԫ�ء�BΪ��Ԫ�ء�CΪ��Ԫ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��2��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ�������ͣ�sp3��
��3��������AC2��CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ
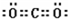 ��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���ʴ�Ϊ��
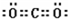 ��N2O��
��N2O����4��EΪCrԪ�أ�ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���������Ų�ʽ�� 1s22s22p63s23p63d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ����ΪHNO3��D�ĵ���ΪMg��HNO3ϡ��Һ��Mg��Ӧʱ��NԪ�ر���ԭ����ͼۣ�������NH4NO3��Mg������ΪMg��NO3��2����NH4NO3��Mg��NO3��2�Ļ�ѧ�������ֱ�Ϊx��y������ݵ���ת���غ���[5-��-3��]��x=2y������x��y=4��1���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��������Ŀ�ۺ��Խϴ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ�����ʽ���������Ų�����������ӻ����ۡ����ӽṹ��������ԭ��Ӧ�ȣ��Ѷ��еȣ������ʽṹ���ۺ�����Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�