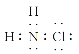��Ŀ����
����Ŀ���л���A����Է�������������150�� ���ⶨA�����������ʣ�
A������ | �ƶ�A����ɡ��ṹ |
����ȼ�գ�ȼ�ղ���ֻ��CO2��H2O | __________ |
��A�봼��������Ũ��������¾�����������ζ������ | __________ |
����һ�������£�A�ܷ�����������ˮ��Ӧ��������������ˮ��ϣ���ˮ��ɫ | |
��0.1 mol A������NaHCO3��Һ��Ӧ����4.48 L(��״����)���� | ___________ |
(1)��д���пհס�
(2)��A��������Ԫ�ص���������Ϊ59.7%����A�ķ���ʽΪ_______________
(3)��A��������̼֧����д����Ӧ�۵Ļ�ѧ����ʽ��_____________________��
(4)����������A��Ϊͬϵ�����________(����ĸ)��
a. ![]() b.
b. ![]()
c. ![]() d.
d. ![]()
���𰸡�һ������C��HԪ�أ����ܺ���OԪ�� A�����к��У�OH����COOH A�����к���������COOH C4H6O5 ![]()
![]() HOOCCH=CHCOOH+H2O b
HOOCCH=CHCOOH+H2O b
��������
����ȼ�գ�ȼ�ղ���ֻ��CO2��H2O������ԭ���غ㣬�л�����һ������C��HԪ�أ������ж��Ƿ���OԪ�أ���A�봼��������Ũ��������¾�����������ζ�����ʣ�˵����Ӧ�������������ʣ���A�к����Ȼ����ǻ�������һ�������£�A�ܷ�����������ˮ��Ӧ��������������ˮ��ϣ���ˮ��ɫ��Ӧ�Ƿ�����ȥ��Ӧ������̼̼˫������A�����ǻ�������̼����λ̼������ԭ�ӣ���0.1mol A������NaHCO3��Һ��Ӧ��˵�����Ȼ�������4.48L(��״����)������̼��˵��A�����к��������Ȼ������A����Է�������С��150������������������Ϊ59.7%���������Oԭ�������Ŀ��![]() =5.6���ݴ˷����ж�A�ķ���ʽ����A��������̼֧�����ݴ˷����ж�A�Ľṹ��ʽ���ݴ˷������
=5.6���ݴ˷����ж�A�ķ���ʽ����A��������̼֧�����ݴ˷����ж�A�Ľṹ��ʽ���ݴ˷������
(1)����ȼ�գ�ȼ�ղ���ֻ��CO2��H2O������ԭ���غ㣬�л�����һ������C��HԪ�أ����ܺ���OԪ�أ��ʴ�Ϊ��һ������C��HԪ�أ����ܺ���OԪ�أ�
��A�봼��������Ũ��������¾�����������ζ�����ʣ�˵����Ӧ�������������ʣ���A�к����Ȼ����ǻ����ʴ�Ϊ��A�����к���-OH��-COOH��
��0.1mol A������NaHCO3��Һ��Ӧ��˵�����Ȼ�������4.48L(��״����)���弴0.2mol������̼��˵��A�����к��������Ȼ����ʴ�Ϊ��A�����к�������-COOH��
(2)����(1)�з���A����2���Ȼ������ǻ���A����Է�������������150������������������Ϊ59.7%���������Oԭ�������Ŀ��![]() =5.6������ຬ��5��Oԭ�ӣ���һ�������£�A�ܷ�����������ˮ��Ӧ��������������ˮ��ϣ���ˮ��ɫ��Ӧ�Ƿ�����ȥ��Ӧ����A�����ǻ�������̼����λ̼������ԭ�ӣ���A��������4��̼ԭ�ӣ�����2���Ȼ���1���ǻ�������5��Oԭ�ӣ�A����Է�������Ϊ
=5.6������ຬ��5��Oԭ�ӣ���һ�������£�A�ܷ�����������ˮ��Ӧ��������������ˮ��ϣ���ˮ��ɫ��Ӧ�Ƿ�����ȥ��Ӧ����A�����ǻ�������̼����λ̼������ԭ�ӣ���A��������4��̼ԭ�ӣ�����2���Ȼ���1���ǻ�������5��Oԭ�ӣ�A����Է�������Ϊ![]() =134��������������̼ԭ�ӣ�4��̼�����ԭ������Ϊ48��5����Ϊ80����128������Ϊ6��������A���ӵķ���ʽΪC4H6O5���ʴ�Ϊ��C4H6O5��
=134��������������̼ԭ�ӣ�4��̼�����ԭ������Ϊ48��5����Ϊ80����128������Ϊ6��������A���ӵķ���ʽΪC4H6O5���ʴ�Ϊ��C4H6O5��
(3)��A��������̼֧������AΪ![]() ����Ӧ�۵Ļ�ѧ����ʽΪ
����Ӧ�۵Ļ�ѧ����ʽΪ![]()
![]() HOOCCH=CHCOOH+H2O���ʴ�Ϊ��
HOOCCH=CHCOOH+H2O���ʴ�Ϊ��![]()
![]() HOOCCH=CHCOOH+H2O��
HOOCCH=CHCOOH+H2O��
(4)ͬϵ����ָ�ṹ���ƣ�����������һ�������ɸ�CH2ԭ���ŵ��л������A(![]() )��Ϊͬϵ���������ֻ�ܺ���2���Ȼ���1���ǻ���ֻ��b��ȷ���ʴ�Ϊ��b��
)��Ϊͬϵ���������ֻ�ܺ���2���Ȼ���1���ǻ���ֻ��b��ȷ���ʴ�Ϊ��b��