��Ŀ����
����Ŀ������������Ԫ��W��X��Y��Z��ԭ�������������ӡ�W��Yͬ���壬X��Zͬ���壬W��X�γɵĶ�Ԫ�����������֣������¾���Һ�壻Y��Z�ĵ��Ӳ�����ͬ���������YWZ��Һ�е�������������Һ���۲쵽���к�ɫ�����������г�������ζ������ų�������˵����ȷ���ǣ� ��
A.YWZ�ǹ��ۻ�����
B.�����̬�⻯��ķе㣺X��Z
C.ԭ�Ӱ뾶��С�����˳��Ϊ��r(W)��r(X)��r(Y)��r(Z)
D.�����ӵ������ԣ�W��Y
���𰸡�B
��������
����������Ԫ��W��X��Y��Z��ԭ�������������ӣ�W��X�γɵĶ�Ԫ�����������֣������¾���Һ�壬��WΪHԪ�أ�XΪO��Ԫ�أ������γɵĶ�Ԫ��������H2O��H2O2�������¾���Һ�壬��W��Yͬ���壬X��Zͬ���壬Y��Z�ĵ��Ӳ�����ͬ����YΪNaԪ�أ�ZΪSԪ�أ��������NaHS��Һ�е�������������Һ���۲쵽���к�ɫ�����������г�������ζ������ų���������Ӧ![]() ���ݴ˷������
���ݴ˷������
��������������֪W��X��Y��Z�ֱ�ΪH��O��Na��S��
A��NaHS����Na+��HS-���ɣ��������ӻ������A����
B��H2O����֮������γ��������H2S����֮�䲻�γ��������������̬�⻯��ķе㣺H2O��H2S����B��ȷ��
C��ͬһ���ڴ�����Ԫ��ԭ�Ӱ뾶���μ�С��ͬһ������ϵ���Ԫ��ԭ�Ӱ뾶����������ԭ�Ӱ뾶��С�����˳��Ϊ��r(W)��r(X)��r(Z)��r(Y)����C����
D��ͬ����Ԫ�ش������£���ԭ������ǿ��������ӵ�������������������ӵ������ԣ�H+��Na+����D����

����Ŀ��ͭ���仯���������ǵ��ճ����������Ź㷺����;���ش��������⣺
(1)ͭ��ͭ�ε���ɫ��ӦΪ��ɫ���ù�����________(�������չ������������������)��
(2)��̬Cuԭ���У��������ռ�ݵ�����ܲ������________����۵��Ӳ�ĵ����Ų�ʽΪ___________��Cu��Ag������IB�壬�۵㣺Cu________Ag (����>������<��)��
(3)[Cu(NH3)4]SO4 �������ӵ����幹����_______������ԭ�ӵĹ���ӻ�����Ϊ_____��[Cu(NH3)4]SO4 ��Cu2����NH3֮���γɵĻ�ѧ����Ϊ_______________��
(4)��Cu���������������Ҵ�������ȩ����ȩ�ٱ����������ᣬ�����ʵ�������ȩ����������������Ŀ��Ϊ_____________��
(5)�ȡ�ͭ����Ԫ�صĵ縺������� CuCl����________(��������������������)�����
Ԫ�� | Cl | Cu |
�縺�� | 3.2 | 1.9 |
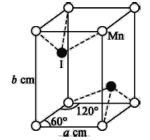
(6)Cu ��Cl �γ�ij�ֻ�����ľ�����ͼ��ʾ���þ�����ܶ�Ϊ�� g��cm��3�������߳�Ϊa cm�����ӵ�����Ϊ______________(�ú�����a�Ĵ���ʽ��ʾ)��