��Ŀ����
�����£���ijһԪ��HA���ס��ҡ�������������ͬ��һԪ�ᣩ��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�Ⱥͻ����Һ��pH���±���ʾ��
��1���Ӽ����������������ж�HA��ǿ�ỹ����� ��
��2����������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ ��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3����������Һ��HA��A-��Na+������Ũ���ɴ�С����Ϊ
��4�������ӷ���ʽ���Ͷ�����Һ�ʼ��Ե�ԭ�� ��Ҫʹ��Һ��
��ֵ������д�ʩ���е���
A���ʵ������¶� B��ͨ��HCl����
C����ˮϡ�� D����������NaA���壨���ܣ�
��5����HAΪ���ᣬһ��Ũ�ȵ�HA��NaA�Ļ����Һ����Ϊ������ϵpH�Ļ�����Һ������Һ�м��������������ҺpH �ı仯��С��������ϵ����Ϊ������Һ����
A����ˮ���Ȼ�炙����Һ B����������Һ
C��������Ȼ��ƻ����Һ D���������Һ
��6��ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2B=H++HB-��HB-?H++B2-����0.1mol?L-1��Na2B��Һ�У�c��B2-��+ =0.1mol?L-1��
| ʵ�� ��� |
HA���ʵ��� Ũ��/��mol?L-1�� |
NaOH���ʵ��� Ũ��/��mol?L-1�� |
��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
��2����������Һ������Ũ��c��A-����c��Na+���Ĵ�С��ϵ
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3����������Һ��HA��A-��Na+������Ũ���ɴ�С����Ϊ
��4�������ӷ���ʽ���Ͷ�����Һ�ʼ��Ե�ԭ��
| c(OH-) |
| c(A-) |
A���ʵ������¶� B��ͨ��HCl����
C����ˮϡ�� D����������NaA���壨���ܣ�
��5����HAΪ���ᣬһ��Ũ�ȵ�HA��NaA�Ļ����Һ����Ϊ������ϵpH�Ļ�����Һ������Һ�м��������������ҺpH �ı仯��С��������ϵ����Ϊ������Һ����
A����ˮ���Ȼ�炙����Һ B����������Һ
C��������Ȼ��ƻ����Һ D���������Һ
��6��ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2B=H++HB-��HB-?H++B2-����0.1mol?L-1��Na2B��Һ�У�c��B2-��+
���㣺���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
��������1�������ʵ������ʱ������ǡ�÷�Ӧ�����Σ�������Һ��pH�ж�����ǿ����
��2���κ���Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��ϵ���غ��жϣ�
��4�������ʵ�������ǡ����ȫ��Ӧ�����ɵ�һNaA����Һ��PH=10˵����Һ�ʼ��ԣ���ˮ������ʼ��ԣ�ˮ��ƽ���ƶ���
��5����������HA��������NaA�Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ�����ʱ����������ʣ������ʱ�������Σ���Һ�������ӻ�����������Ũ�ȱ仯������������ã�
��6�����ݶ�Ԫ��ĵ��뷽��ʽ֪��B2-ֻ������һ��ˮ�⣬��������غ�������
��2���κ���Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��ϵ���غ��жϣ�
��4�������ʵ�������ǡ����ȫ��Ӧ�����ɵ�һNaA����Һ��PH=10˵����Һ�ʼ��ԣ���ˮ������ʼ��ԣ�ˮ��ƽ���ƶ���
��5����������HA��������NaA�Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ�����ʱ����������ʣ������ʱ�������Σ���Һ�������ӻ�����������Ũ�ȱ仯������������ã�
��6�����ݶ�Ԫ��ĵ��뷽��ʽ֪��B2-ֻ������һ��ˮ�⣬��������غ�������
���
�⣺��1����HA��ǿ�ᣬǡ����NaOH��Һ��Ӧ����ǿ��ǿ���Σ�pH=7����HA�����ᣬ���ɵ�NaAˮ���Լ��ԣ�pH��7��
�ʴ�Ϊ��a=7ʱ��HA��ǿ�a��7ʱ��HA�����
��2�������Һ�д��ڵ���غ�c��Na+��+c��H+��=c��A-��+c��OH-��������pH=7����c��Na+��=c��A-�����ʴ�Ϊ��C��
��3����������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��������Ũ���ɴ�С��˳��Ϊ��c��HA����c��Na+����c��A-����c��OH-����c��H+�����ʴ�Ϊ��c��HA����c��Na+����c��A-����
��4�������ʵ�������ǡ����ȫ��Ӧ�����ɵ�һNaA����Һ��PH=10˵����Һ�ʼ��ԣ���A-+H2O?HA+OH-����Ҫʹ��Һ��
��ֵ���ʵ�ʾ���ˮ��ƽ�������ƶ���ԽϡԽˮ�⣬Խ��Խˮ�⣬����AC��ȷ���ʴ�Ϊ��A-+H2O?HA+OH-��AC��
��5�����а�ˮ���Ȼ�淋Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ����ʱ����������ʣ�������ʱ�������Σ������ڼ�����ʱ������NH3?H2O+H+?NH4++H2O�������ʱ������NH4++OH-?NH3?H2O����Һ�������ӻ�����������Ũ�ȱ仯������������ã��ʴ�Ϊ��A��
��6����Na2B�д���ˮ��ƽ�⣺B2-+H2O=HB-+OH-��HB-�����һ��ˮ�⣬������Һ��û��H2B���ӣ����������غ��c��B2-��+c��HB-��=0.1mol?L-1��
�ʴ�Ϊ��c��HB-����
�ʴ�Ϊ��a=7ʱ��HA��ǿ�a��7ʱ��HA�����
��2�������Һ�д��ڵ���غ�c��Na+��+c��H+��=c��A-��+c��OH-��������pH=7����c��Na+��=c��A-�����ʴ�Ϊ��C��
��3����������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��������Ũ���ɴ�С��˳��Ϊ��c��HA����c��Na+����c��A-����c��OH-����c��H+�����ʴ�Ϊ��c��HA����c��Na+����c��A-����
��4�������ʵ�������ǡ����ȫ��Ӧ�����ɵ�һNaA����Һ��PH=10˵����Һ�ʼ��ԣ���A-+H2O?HA+OH-����Ҫʹ��Һ��
| c(OH-) |
| c(A-) |
��5�����а�ˮ���Ȼ�淋Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ����ʱ����������ʣ�������ʱ�������Σ������ڼ�����ʱ������NH3?H2O+H+?NH4++H2O�������ʱ������NH4++OH-?NH3?H2O����Һ�������ӻ�����������Ũ�ȱ仯������������ã��ʴ�Ϊ��A��
��6����Na2B�д���ˮ��ƽ�⣺B2-+H2O=HB-+OH-��HB-�����һ��ˮ�⣬������Һ��û��H2B���ӣ����������غ��c��B2-��+c��HB-��=0.1mol?L-1��
�ʴ�Ϊ��c��HB-����
���������⿼����������ʵĵ��롢����Ũ�ȴ�С�ıȽϣ���ȷ������ʵ����ص��������غ㡢����غ�������غ����������ͬʱע�����Һ��ԭ����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ������֪���⻯������Ϊ-1�ۣ����йظõ�ص�˵����ȷ���ǣ�������
����NaBH4/H2O2ȼ�ϵ�أ�DBFC���Ľṹ��ͼ��ʾ������֪���⻯������Ϊ-1�ۣ����йظõ�ص�˵����ȷ���ǣ�������| A���缫B�����к�MnO2�㣬MnO2����ǿ������ |
| B����ظ������ĵ缫��Ӧ��BH4-+8OH--8e-�TBO2-+6H2O |
| C���ŵ�����У�Na+������������Ǩ�� |
| D���ڵ�ط�Ӧ�У�ÿ����1L 6mol/L H2O2��Һ��������������·�еĵ���Ϊ6NA�� |
��ɫ��ѧ�ĺ��������û�ѧԭ����Դͷ�ϼ��ٺ�������ҵ�����Ի�������Ⱦ��������ġ�ԭ�Ӿ��á�����ԭ��������Ϊ100%��������ȡ�л���ķ�Ӧ��ԭ����������͵��ǣ�������
A��nCH2=CH2
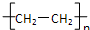 | ||
B��CH2=CH2+HCl
| ||
C��C6H12O6
| ||
D�� |
 �ڻ�ѧ��Ӧ�У�ֻ������������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ���ܷ�����ѧ��Ӧ�����ǰ���������ѧ��Ӧ����ײ��Ϊ��Ч��ײ��������Ч��ײ�ķ��ӳ�Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λ��kJ?mol-1��ʾ������۲���ͼ��Ȼ��ش����⣮
�ڻ�ѧ��Ӧ�У�ֻ������������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ���ܷ�����ѧ��Ӧ�����ǰ���������ѧ��Ӧ����ײ��Ϊ��Ч��ײ��������Ч��ײ�ķ��ӳ�Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λ��kJ?mol-1��ʾ������۲���ͼ��Ȼ��ش����⣮