��Ŀ����
��12�֣��������к��д����ơ�����þ�ͼص�Ԫ�ء�ʵ���ҿ���ͼʾ���̲ⶨ�������иơ�þԪ�غ��������ݵķ�Ӧ���Ա�ʾΪ��Ca2++Y2-=CaY��Mg2++Y2-=MgY���ش��������⡣
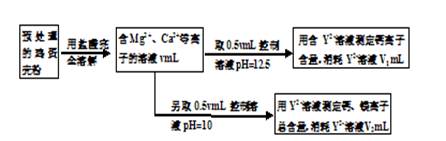
��1���ⶨCa2+��Mg2+�ܺ���ʱ��������ҺpH=10����pH���ⶨ����� ���ƫ����ƫС������Ӱ�족����
��2���ⶨCa2+�����Ĺؼ��ǿ�����Һ��pH��ʹ��Һ��Mg2+�γɳ�������Ҫʹ��Һ��c(Mg2+)������1.2��10-7mol/L������ҺpHӦ��С�� (��֪��Ksp[Mg(OH)2]=1.2��10��11������ʵ����������½���)��
��3����������Ƿ�����Ϊmg����Һ��Y2+Ũ��Ϊc mol/L��������Ʒ��þԪ������������ ��
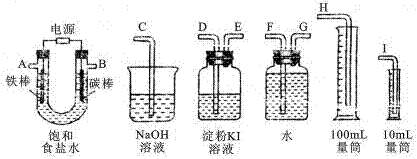
��4����ͬѧ����ɲ�������װ�á�ͨ���ⶨ�����������ᷴӦ�����Ķ�����̼�������ܲⶨ�������еĸƵ��ܺ�����

�ٲ��ø÷�������ʵ�飬��װ�ô����ҵ�����˳�����Σ�
1��2��__ ________________�����װ�ö�Ӧ�ӿڵ�������ţ���
�����ų�ʵ�������Ͳ�����Ӱ�����أ���ʵ�鷽����õĽ���Ƿ�ȷ��
��________���ȷ��������һ��ȷ�������жϡ�����
ԭ����___________________ __
��

��1���ⶨCa2+��Mg2+�ܺ���ʱ��������ҺpH=10����pH���ⶨ����� ���ƫ����ƫС������Ӱ�족����
��2���ⶨCa2+�����Ĺؼ��ǿ�����Һ��pH��ʹ��Һ��Mg2+�γɳ�������Ҫʹ��Һ��c(Mg2+)������1.2��10-7mol/L������ҺpHӦ��С�� (��֪��Ksp[Mg(OH)2]=1.2��10��11������ʵ����������½���)��
��3����������Ƿ�����Ϊmg����Һ��Y2+Ũ��Ϊc mol/L��������Ʒ��þԪ������������ ��
��4����ͬѧ����ɲ�������װ�á�ͨ���ⶨ�����������ᷴӦ�����Ķ�����̼�������ܲⶨ�������еĸƵ��ܺ�����

�ٲ��ø÷�������ʵ�飬��װ�ô����ҵ�����˳�����Σ�
1��2��__ ________________�����װ�ö�Ӧ�ӿڵ�������ţ���
�����ų�ʵ�������Ͳ�����Ӱ�����أ���ʵ�鷽����õĽ���Ƿ�ȷ��
��________���ȷ��������һ��ȷ�������жϡ�����
ԭ����___________________ __
��
��12�֣���1��ƫС��2�֣� ��2��12 ��2�֣� ��3��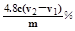 ��3�֣�
��3�֣�
��4����6��7��4��5��3��6��7��5��4��3��2�֣��ڲ�һ��ȷ��
��1�֣���������и�Ԫ��ȫ����̼�����ʽ���ڣ��Ҳ��ٺ����������ᷴӦ��������������������ʣ���ⶨ���ȷ������ȷ��
�����������⣬ֻҪ������������֣���2�֣�
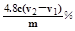 ��3�֣�
��3�֣���4����6��7��4��5��3��6��7��5��4��3��2�֣��ڲ�һ��ȷ��
��1�֣���������и�Ԫ��ȫ����̼�����ʽ���ڣ��Ҳ��ٺ����������ᷴӦ��������������������ʣ���ⶨ���ȷ������ȷ��
�����������⣬ֻҪ������������֣���2�֣�
��1�����pH��������Mg(OH)2�������Ӷ�ʹ����Y2-�����ƫС���ⶨ���ƫС��
��2��Ksp=c2(OH-)c(Mg2+)��c(OH-)=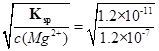 =0.01mol/L����pH����12��
=0.01mol/L����pH����12��
��3��þ����Y2-�����Ϊ��V2-V1��ml��n(Mg2+)=c����V2-V1����10-3mol��m(Mg2+)=c����V2-V1����10-3��24g������������Ϊ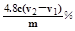
��4���ٷ�Ӧ�����Ũ���ᣩ������CO2����ʯ�ң�����ֹ������ˮ��������̼���룻�ʴ�Ϊ6��7��4��5��3��6��7��5��4��3��
�ڲ�һ��ȷ�����ܱ�֤��Ԫ��ȫ����̼�����ʽ���ڣ��Ҳ��ٺ����������ᷴӦ��������������������ʣ���ⶨ���ȷ������ȷ��
��2��Ksp=c2(OH-)c(Mg2+)��c(OH-)=
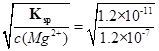 =0.01mol/L����pH����12��
=0.01mol/L����pH��С��12����3��þ����Y2-�����Ϊ��V2-V1��ml��n(Mg2+)=c����V2-V1����10-3mol��m(Mg2+)=c����V2-V1����10-3��24g������������Ϊ
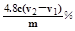
��4���ٷ�Ӧ�����Ũ���ᣩ������CO2����ʯ�ң�����ֹ������ˮ��������̼���룻�ʴ�Ϊ6��7��4��5��3��6��7��5��4��3��
�ڲ�һ��ȷ�����ܱ�֤��Ԫ��ȫ����̼�����ʽ���ڣ��Ҳ��ٺ����������ᷴӦ��������������������ʣ���ⶨ���ȷ������ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ