��Ŀ����
ͼ��ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺

��1���ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
��2������Ԫ�ؿ��γɶ��ֻ�������мȺ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����ĵ���ʽΪ
 ��
��
��3��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
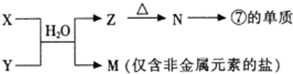
��4����ͼ����Ԫ���γɵij�������X��Y��Z��M��N�ɷ���ͼ����ʾ��Ӧ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽ
N���ĵ��ʵĻ�ѧ����ʽΪ
M�������ӵļ�������

��1���ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ�����������
ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ�����������
��ԭ�Ӱ뾶�����õ��������������ǽ�������������2������Ԫ�ؿ��γɶ��ֻ�������мȺ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����ĵ���ʽΪ


��3��W��������ڵ�ͬ����Ԫ�أ����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| 1 | ||
| 2 |
X��Һ��Y��Һ��Ӧ�����ӷ���ʽ
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
Al3++3NH3?H2O�TAl��OH��3��+3NH4+
��N���ĵ��ʵĻ�ѧ����ʽΪ
2Al2O3�����ڣ�
4Al+3O2��
| ||
| ��� |
2Al2O3�����ڣ�
4Al+3O2��
��
| ||
| ��� |
M�������ӵļ�������
ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����
ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠�����
����������1���ɼ�ͼ��֪��Ϊ̼Ԫ�ء���Ϊ��Ԫ�أ�������������ͬ�����ԭ�Ӱ뾶���õ��������������ǽ�����������
��2���ɼ�ͼ�б�ע��Ԫ�ؿ�֪���Ⱥ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����Ϊ�������ƣ�
��3��W��������ڵ�ͬ����Ԫ�أ��ɼ�ͼ��֪������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ�
��4��MΪֻ���ǽ���Ԫ�ص��Σ���MΪ��Σ���ΪAl��������ڵ�Al2O3�ɵ�Al����ת����ϵ��������֪��NΪAl2O3��ZΪAl��OH��3������X��YΪ��ˮ�����Σ�����笠����ӣ������ת��Ϊ����������ʹʪ��ĺ�ɫʯ����ֽ������
��2���ɼ�ͼ�б�ע��Ԫ�ؿ�֪���Ⱥ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����Ϊ�������ƣ�
��3��W��������ڵ�ͬ����Ԫ�أ��ɼ�ͼ��֪������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ�
��4��MΪֻ���ǽ���Ԫ�ص��Σ���MΪ��Σ���ΪAl��������ڵ�Al2O3�ɵ�Al����ת����ϵ��������֪��NΪAl2O3��ZΪAl��OH��3������X��YΪ��ˮ�����Σ�����笠����ӣ������ת��Ϊ����������ʹʪ��ĺ�ɫʯ����ֽ������
����⣺��1���ɼ�ͼ��֪��Ϊ̼Ԫ�ء���Ϊ��Ԫ�أ�C��Si������������ͬ��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ����������࣬ԭ�Ӱ뾶�����õ��������������ǽ����Լ�������������̼��ǿ�ڹ��ᣮ
�ʴ�Ϊ��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ����������࣮
��2����������������������������ӹ��ɣ�����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
�ɼ�ͼ�б�ע��Ԫ�ؿ�֪���Ⱥ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����Ϊ�������ƣ�
��3��W��������ڵ�ͬ����Ԫ�أ��ɼ�ͼ��֪������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ�
�ʴ�Ϊ�� ��
��
��4��MΪֻ���ǽ���Ԫ�ص��Σ���MΪ��Σ���ΪAl��������ڵ�Al2O3�ɵ�Al����ת����ϵ��������֪��NΪAl2O3��ZΪAl��OH��3������X��YΪ��ˮ�����Σ�
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
������ڵ�Al2O3�ɵ�Al��N���ĵ��ʵĻ�ѧ����ʽΪ2Al2O3�����ڣ�
4Al+3O2����
����笠����ӣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��2Al2O3�����ڣ�
4Al+3O2����ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
�ʴ�Ϊ��ͬһ����Ԫ�ش��ϵ���ԭ�Ӻ�����Ӳ����������࣮
��2����������������������������ӹ��ɣ�����ʽΪ
 ��
���ʴ�Ϊ��
 ��
���ɼ�ͼ�б�ע��Ԫ�ؿ�֪���Ⱥ����Ӽ��ֺ��Ǽ��Թ��ۼ��Ļ�����Ϊ�������ƣ�
��3��W��������ڵ�ͬ����Ԫ�أ��ɼ�ͼ��֪������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ�
�ʴ�Ϊ��
 ��
����4��MΪֻ���ǽ���Ԫ�ص��Σ���MΪ��Σ���ΪAl��������ڵ�Al2O3�ɵ�Al����ת����ϵ��������֪��NΪAl2O3��ZΪAl��OH��3������X��YΪ��ˮ�����Σ�
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O�TAl��OH��3��+3NH4+��
������ڵ�Al2O3�ɵ�Al��N���ĵ��ʵĻ�ѧ����ʽΪ2Al2O3�����ڣ�
| ||
| ��� |
����笠����ӣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬
�ʴ�Ϊ��Al3++3NH3?H2O�TAl��OH��3��+3NH4+��2Al2O3�����ڣ�
| ||
| ��� |
����������Ԫ�����ڱ��������ƶϡ�Ԫ�ػ��������ʡ����û�ѧ����ȣ��Ѷ��еȣ�����Ԫ�ػ�������������ƶϵĹؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ��

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д� ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
�����Ŀ