��Ŀ����
��2013?����һģ����ͼΪԪ�����ڱ���һ���֣������Ԫ�آ�һ���ڱ��е�λ�ã��ش���������
��1��Ԫ�آ��γɵļ������ӵĽṹʾ��ͼΪ
 ��Ԫ�آܡ��ޡ����γɵļ����ӵİ뾶�ɴ�С��˳����
��Ԫ�آܡ��ޡ����γɵļ����ӵİ뾶�ɴ�С��˳����
��2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ��������������Ԫ�آܵĵ��ʰ����ʵ���֮��Ϊ4��1ͨ�뺬������Ԫ�آ١��ܺ͢���ɵĻ������ҵ�ˮ��Һ�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣��䷴Ӧ�Ļ�ѧ����ʽΪ
��3��Ԫ�آں͢���ɵ�һ�ֻ�������Ԫ�آܵĵ��ʺͻ������ҵ�ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ�д����ȼ�ϵ�ظ����ĵ缫��Ӧʽ
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
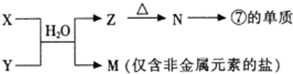
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ
��N���ߵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
��M��ˮ��Һ�����ԣ������ӷ���ʽ������ԭ��
| �� | ��A | 0 | ||||||
| ���� | ||||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ����A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | |||||


O2-��Na+��Al3+
O2-��Na+��Al3+
�������ӷ��ű�ʾ������2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ��������������Ԫ�آܵĵ��ʰ����ʵ���֮��Ϊ4��1ͨ�뺬������Ԫ�آ١��ܺ͢���ɵĻ������ҵ�ˮ��Һ�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣��䷴Ӧ�Ļ�ѧ����ʽΪ
4NO+O2+4NaOH=4NaNO2+2H2O
4NO+O2+4NaOH=4NaNO2+2H2O
����3��Ԫ�آں͢���ɵ�һ�ֻ�������Ԫ�آܵĵ��ʺͻ������ҵ�ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ�д����ȼ�ϵ�ظ����ĵ缫��Ӧʽ
CO+4OH-+2e-=CO32-+2H2O
CO+4OH-+2e-=CO32-+2H2O
����4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ
Al3++3NH3?H2O=Al��OH��3��+3NH4+
Al3++3NH3?H2O=Al��OH��3��+3NH4+
����N���ߵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
2Al2O3
4Al+3O2��
| ||
2Al2O3
4Al+3O2��
��
| ||
��M��ˮ��Һ�����ԣ������ӷ���ʽ������ԭ��
NH4++H2O?NH3?H2O+H+
NH4++H2O?NH3?H2O+H+
������������Ԫ���������ڱ��е�λ�ÿ�֪����ΪHԪ�أ���ΪCԪ�أ���ΪNԪ�أ���ΪOԪ�أ���ΪFԪ�أ���ΪNaԪ�أ���ΪAlԪ�أ���ΪClԪ�أ�
��1��Cl-����������Ϊ17�����������Ϊ18����3�����Ӳ㣬���������Ϊ2��8��8��
�ܡ��ޡ����γɵļ����ӷֱ�ΪO2-��Na+��Al3+�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС��
��2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ����ΪNO����Ԫ�آ١��ܺ͢���ɵĻ������ң���ΪNaOH��NO��O2�����ʵ���֮��Ϊ4��1ͨ�뺬����NaOH�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣�NԪ�ر����������ݵ���ת��ȷ��NԪ�������еĻ��ϼۣ��ݴ���д��
��3��Ԫ�آں͢���ɵ�һ�ֻ�������O2��NaOH��ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ���Ԫ�آں͢���ɵĻ�����ΪCO��ԭ��ظ�������������Ӧ��CO�ڸ����ŵ磬��������������CO32-��H2O��
��4��M�ǽ����ǽ�����������һ������Σ���Һ��NH4+����ˮ�⣬�ƻ�ˮ�ĵ���ƽ�⣬ˮ��Һ�����ԣ�
Z���ߵĵ��ʣ���ΪAlԪ�����������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ��
��1��Cl-����������Ϊ17�����������Ϊ18����3�����Ӳ㣬���������Ϊ2��8��8��
�ܡ��ޡ����γɵļ����ӷֱ�ΪO2-��Na+��Al3+�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС��
��2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ����ΪNO����Ԫ�آ١��ܺ͢���ɵĻ������ң���ΪNaOH��NO��O2�����ʵ���֮��Ϊ4��1ͨ�뺬����NaOH�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣�NԪ�ر����������ݵ���ת��ȷ��NԪ�������еĻ��ϼۣ��ݴ���д��
��3��Ԫ�آں͢���ɵ�һ�ֻ�������O2��NaOH��ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ���Ԫ�آں͢���ɵĻ�����ΪCO��ԭ��ظ�������������Ӧ��CO�ڸ����ŵ磬��������������CO32-��H2O��
��4��M�ǽ����ǽ�����������һ������Σ���Һ��NH4+����ˮ�⣬�ƻ�ˮ�ĵ���ƽ�⣬ˮ��Һ�����ԣ�
Z���ߵĵ��ʣ���ΪAlԪ�����������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ��
����⣺����Ԫ���������ڱ��е�λ�ÿ�֪����ΪHԪ�أ���ΪCԪ�أ���ΪNԪ�أ���ΪOԪ�أ���ΪFԪ�أ���ΪNaԪ�أ���ΪAlԪ�أ���ΪClԪ�أ�
��1��Cl-����������Ϊ17�����������Ϊ18����3�����Ӳ㣬���������Ϊ2��8��8���������ӵĽṹʾ��ͼΪ�� ��
��
�ܡ��ޡ����γɵļ����ӷֱ�ΪO2-��Na+��Al3+�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��O2-��Na+��Al3+��
�ʴ�Ϊ�� ��O2-��Na+��Al3+��
��O2-��Na+��Al3+��
��2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ����ΪNO����Ԫ�آ١��ܺ͢���ɵĻ������ң���ΪNaOH��NO��O2�����ʵ���֮��Ϊ4��1ͨ�뺬����NaOH�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣�NԪ�ر���������NԪ�������еĻ��ϼ�Ϊa���ɵ���ת���غ��֪4��a-2��=1��2��[0-��-2��]�����a=3�������ɵ���ΪNaNO2����Ӧ����ʽΪ4NO+O2+4NaOH=4NaNO2+2H2O��
�ʴ�Ϊ��4NO+O2+4NaOH=4NaNO2+2H2O��
��3��Ԫ�آں͢���ɵ�һ�ֻ�������O2��NaOH��ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ���Ԫ�آں͢���ɵĻ�����ΪCO��ԭ��ظ�������������Ӧ��CO�ڸ����ŵ磬��������������CO32-��H2O�������缫��ӦʽΪCO+4OH--2e-=CO32-+2H2O��
�ʴ�Ϊ��CO+4OH--2e-=CO32-+2H2O��
��4��M�ǽ����ǽ�����������һ������Σ�Z���ߵĵ��ʣ���ΪAlԪ�����������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ��
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ڹ�ҵ���õ��Al2O3�ķ���ұ��Al��N���ߵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ��2Al2O3
4Al+3O2�����ʴ�Ϊ��2Al2O3
4Al+3O2����
��M����Σ���Һ��NH4+����ˮ��NH4++H2O?NH3?H2O+H+���ƻ�ˮ�ĵ���ƽ�⣬ˮ��Һ�����ԣ��ʴ�Ϊ��NH4++H2O?NH3?H2O+H+��
��1��Cl-����������Ϊ17�����������Ϊ18����3�����Ӳ㣬���������Ϊ2��8��8���������ӵĽṹʾ��ͼΪ��
 ��
���ܡ��ޡ����γɵļ����ӷֱ�ΪO2-��Na+��Al3+�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶��O2-��Na+��Al3+��
�ʴ�Ϊ��
 ��O2-��Na+��Al3+��
��O2-��Na+��Al3+����2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ����ΪNO����Ԫ�آ١��ܺ͢���ɵĻ������ң���ΪNaOH��NO��O2�����ʵ���֮��Ϊ4��1ͨ�뺬����NaOH�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣�NԪ�ر���������NԪ�������еĻ��ϼ�Ϊa���ɵ���ת���غ��֪4��a-2��=1��2��[0-��-2��]�����a=3�������ɵ���ΪNaNO2����Ӧ����ʽΪ4NO+O2+4NaOH=4NaNO2+2H2O��
�ʴ�Ϊ��4NO+O2+4NaOH=4NaNO2+2H2O��
��3��Ԫ�آں͢���ɵ�һ�ֻ�������O2��NaOH��ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ���Ԫ�آں͢���ɵĻ�����ΪCO��ԭ��ظ�������������Ӧ��CO�ڸ����ŵ磬��������������CO32-��H2O�������缫��ӦʽΪCO+4OH--2e-=CO32-+2H2O��
�ʴ�Ϊ��CO+4OH--2e-=CO32-+2H2O��
��4��M�ǽ����ǽ�����������һ������Σ�Z���ߵĵ��ʣ���ΪAlԪ�����������ƶ�N������������Z���������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪���÷�ӦΪ���κ�һˮ�ϰ��ķ�Ӧ��
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
�ڹ�ҵ���õ��Al2O3�ķ���ұ��Al��N���ߵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ��2Al2O3
| ||
| ||
��M����Σ���Һ��NH4+����ˮ��NH4++H2O?NH3?H2O+H+���ƻ�ˮ�ĵ���ƽ�⣬ˮ��Һ�����ԣ��ʴ�Ϊ��NH4++H2O?NH3?H2O+H+��
���������⿼��������ƶϼ�Ԫ�������ɡ�Ԫ�����ڱ���������ԭ��Ӧ��ԭ��ء�����ˮ�⣬Ԫ�ؼ����ʵ��ƶ��ǽ����Ĺؼ���ע�ضԸ߿���������Ŀ��飬��ѧ������Ҫ��ϸߣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
