��Ŀ����
��1�����1gˮ�к���n����ԭ�ӣ����ӵ�������
��2����˹�м��飨CH4����������������Ϊ1��4ʱ���ױ�ը�����ʱ�����������������Ϊ ��
��3��SO2��O2�Ļ�������У���Ԫ�ص���������Ϊ70%����SO2��O2�����ʵ���֮���� �����ֻ��������ܶ���ͬ��ͬѹ�������ܶȵ� ����
��4����̬������A�Ļ�ѧʽ���Ա�ʾΪOxFy����֪ͬ��ͬѹ��10mLA������ȫ�ֽ�����15mLO2��10mL F2����A�Ļ�ѧʽΪ ��
��2����˹�м��飨CH4����������������Ϊ1��4ʱ���ױ�ը�����ʱ�����������������Ϊ
��3��SO2��O2�Ļ�������У���Ԫ�ص���������Ϊ70%����SO2��O2�����ʵ���֮����
��4����̬������A�Ļ�ѧʽ���Ա�ʾΪOxFy����֪ͬ��ͬѹ��10mLA������ȫ�ֽ�����15mLO2��10mL F2����A�Ļ�ѧʽΪ
���㣺���ʵ�������ؼ���,��ѧ����ʽ���йؼ���,�йػ���ﷴӦ�ļ���
ר�⣺������
��������1��1��ˮ��������1����ԭ�Ӻ�2����ԭ�ӹ��ɵģ�����ˮ���ӵĹ��ɡ�n=
��N=n��NA���㼴�ɽ�𣮣�2������V=
Vm���㣻
��3���������������Ϊ100g��������Ԫ�ص�������������SԪ��������������������SԪ���������ټ���SO2������������������������n=
����������ʵ���������
=
����ƽ����Է�����������ͬ�����£���ͬ���ʵ��ܶ�֮�ȵ�����Ħ������֮�ȣ�
��4�����������غ㶨�ɺͰ����ӵ����ɣ�д���ֽⷽ��ʽΪ2OxFy
3O2+2F2������ƶϻ�ѧʽ��
| m |
| M |
| m |
| M |
��3���������������Ϊ100g��������Ԫ�ص�������������SԪ��������������������SԪ���������ټ���SO2������������������������n=
| m |
| M |
. |
| M |
| m(��) |
| n(��) |
��4�����������غ㶨�ɺͰ����ӵ����ɣ�д���ֽⷽ��ʽΪ2OxFy
| ||
���
�⣺��1��1gˮ�����ʵ���n=
=
=
mol��1��ˮ��������1����ԭ�Ӻ�2����ԭ�ӹ��ɵģ���1gˮ����ԭ�ӵ����ʵ���Ϊ
mol��2=
mol����N=n��NA��֪��n=
mol��NA�������ӵ������ɱ�ʾΪNA=
=9nmol-1���ʴ�Ϊ��9nmol-1��
��2������V=
Vm֪����ͬ�����£�����Ħ�������ȣ������֮�ȵ��������ʵ���֮��=
��
=1��2���ʴ�Ϊ��1��2��
��3��SO2��O2�Ļ�������У���Ԫ�ص���������Ϊ70%����SԪ�ص���������=1-70%=30%���������������Ϊ100g����SԪ������=100g��30%=30g����SO2������=30g��
=60g��������������=100g-60g=40g����SO2�����ʵ���=
=
mol��O2�����ʵ���=
=
mol����SO2��O2�����ʵ���֮��Ϊ
mol��
mol=3��4������ƽ����Է�������=
=45.71����ͬ�����£������Ħ������֮�ȵ��������ʵ���֮�ȣ����Ի��������ܶ���ͬ��ͬѹ�������ܶȵı���=
=
��
�ʴ�Ϊ��3��4��
��
��4�����������غ㶨�ɺͰ����ӵ����ɣ�д���ֽⷽ��ʽΪ2OxFy
3O2+2F2����A��ѧʽΪO3F2���ʴ�Ϊ��O3F2��
| m |
| M |
| 1g |
| 18g/mol |
| 1 |
| 18 |
| 1 |
| 18 |
| 1 |
| 9 |
| 1 |
| 9 |
| n | ||
|
��2������V=
| m |
| M |
| 1 |
| 16 |
| 4 |
| 32 |
��3��SO2��O2�Ļ�������У���Ԫ�ص���������Ϊ70%����SԪ�ص���������=1-70%=30%���������������Ϊ100g����SԪ������=100g��30%=30g����SO2������=30g��
| 32 |
| 64 |
| 60g |
| 64g/mol |
| 15 |
| 16 |
| 40g |
| 32g/mol |
| 20 |
| 16 |
| 15 |
| 16 |
| 20 |
| 16 |
| 100 | ||||
|
| 45.71 |
| 32 |
| 10 |
| 7 |
�ʴ�Ϊ��3��4��
| 10 |
| 7 |
��4�����������غ㶨�ɺͰ����ӵ����ɣ�д���ֽⷽ��ʽΪ2OxFy
| ||
���������⿼�������ʵ������йؼ��㼰��ѧʽ��ȷ������ȷ����֮��ķ�Ӧ��������ʽ�ǽⱾ��ؼ���֪����ͬ�����²�ͬ�����ܶ���Ħ�������Ĺ�ϵ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
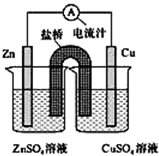 ��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������
��ͼΪһԭ��ص�װ��ʾ��ͼ������˵������ȷ���ǣ�������| A��ԭ��ع���ʱ���ܷ�ӦΪ��Zn+Cu2+=Zn2++Cu |
| B��ԭ��ع���ʱ��������ͭ����������������п�� |
| C��ԭ��ع���ʱ��������K+����CuSO4��Һ |
| D�������Cu�缫��Zn�缫������������ָ�뷴��ƫת |
�йص���ʵ�˵����ȷ���ǣ�������
| A��FeCl3��Һ�ܹ����磬����FeCl3��Һ�ǵ���� |
| B��CO2ˮ��Һ�ܹ����磬����CO2�ǵ���� |
| C��Һ̬��ͭ�����Ժܺã�����ͭ�ǵ���� |
| D��NaOH��������ˮ���ܵ��磬����NaOH�ǵ���� |
���и�ͼ����ʾ�ķ�Ӧ�����ȷ�Ӧ���ǣ�������
A��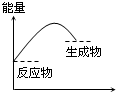 ��Ӧ���� |
B��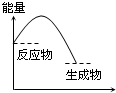 ��Ӧ���� |
C��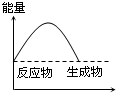 ��Ӧ���� |
D��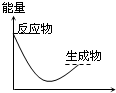 ��Ӧ���� |
��NAΪ�����ӵ������������й�˵����ȷ���ǣ�������
| A��0.5mol�������к���C=C˫����Ϊ1.5NA | ||
| B��2.8g��ϩ�ͱ�ϩ�Ļ������������̼ԭ����Ϊ0.2NA | ||
C����״���£�1L�״���ȫȼ�պ����ɵ�CO2������ĿԼΪ
| ||
| D��1 mol����-CH3�������ĵ�������Ϊ8 NA |