��Ŀ����
�������ʵ���������ͭ�Ļ�̨��24 g��600mLϡ����ǡ����ȫ��Ӧ������NO6 .72 L(��״������Ӧ�����Һ�м���l mol��L-1 NaOH��Һʹ��������ǡ�ó��������ˡ������й�˵���������
| A��������ܽ�����Һ�� c(Fe3��)�� c(Fe2+) =1��1 |
| B�������NaOH��Һ1000mL |
| C��ϡ��������ʵ���Ũ����2 mol��L-1 |
| D��������ó����ڿ����г�ּ��ȿɵù���32 g |
B
�������������������ͭ�����ʵ�����Ϊxmol����56x+64x=24��x=0.2,��Ӧ��Cuʧȥ���ӵ����ʵ�����0.2mol��2=0.4mol�����÷�Ӧ�й�ת�Ƶ��ӵ����ʵ�����6.72L/22.4L/mol��3=0.9mol������0.2molFeʧȥ���ӵ����ʵ���Ӧ��0.9-0.4=0.5mol�����Է�Ӧ�����Һ�м��������������������ӣ��Ҷ��ߵ����ʵ���0.1mol�����Է�Ӧ�����Һ����0.1mol Fe3+��0.1molFe2+��0.2molCu2+��A��������ܽ�����Һ�� c(Fe3��)�� c(Fe2+) =1��1 ����ȷ��B����Һ�����������Ӵ��ڣ�˵���������꣬�����������������Ƶ����ʵ�����0.1mol��3+0.1mol��2+0.2mol��2=0.9mol����Ҫl mol��L-1�������Ƶ������900mL������C������NԪ���غ㣬ԭ��Һ����������ʵ�����0.1mol��3+0.1mol��2+0.2mol��2+6.72L/22.4L/mol=1.2mol������ϡ��������ʵ���Ũ����1.2mol/0.6L="2" mol��L-1����ȷ��D�������ڼ��ȹ������������ӱ�����Ϊ�����ӣ�����������ó����ڿ����г�ּ��ȵõ�������������ͭ�Ĺ��壬����ͭ�����ʵ�����0.2mol�������������ʵ�����0.1mol�����Թ��������������32g����ȷ����ѡB��
���㣺��������ͭ�Ļ�ѧ���ʣ�������ԭ��Ӧ�ļ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺
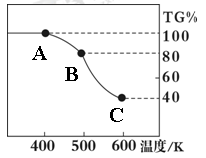
��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
MnO2��һЩ���ʻ���;��ͼ������˵����ȷ���� ( )
| A���١��ڡ���������Ӧ��MnO2���������� |
| B������MnO2��2 L 10 mol/LHCl���ȣ�������5 mol C12 |
| C����Ӧ��������1 mol Al2O3����Ӧ������ת��12 mol���� |
| D����Ӧ����K2CO3��KNO3�Ļ�ѧ��������Ϊ1 |
���ݱ�����Ϣ�жϣ�����ѡ����ȷ���� �� ��
| ��� | ��Ӧ�� | ���� |
| �� | KMnO4��H2O2��H2SO4 | K2SO4��MnSO4���� |
| �� | Cl2��FeBr2 | FeCl3��FeBr3 |
| �� | MnO4������ | Cl2��Mn2+���� |
B���ڢ��鷴Ӧ��Cl2��FeBr2�����ʵ���֮�ȴ��ڻ����1:2
C���ڢ��鷴Ӧ������1mol Cl2��ת�Ƶ���5mol
D����������ǿ����˳��ΪMnO4��>Cl2> Br2> Fe3+
�����£���ˮ��Һ�з������·�Ӧ���� ��
��16H++10C��+2XO4 �� = 2X2+��5C2��8H2O����2A2+��B2 = 2A3+��2B������2B����C2 = B2��2C����
����˵���������
| A����ӦC2 �� 2A2+ = 2A3+ �� 2C�� ���Խ��� |
| B����ԭ����ǿ������˳����C��>A2+>B��>X2+ |
| C����������ǿ������˳����XO4��>C2>B2>A3+ |
| D����Ӧ�����û���Ӧ |
ij�Ͻ�(����ͭ����)��ͭ���������ʵ���֮��Ϊymol������Cu�����ʵ�������Ϊa ������ȫ��Ͷ��50mLbmol��L��1��������Һ�У�����ʹ���ַ�Ӧ(����NO��Ψһ�Ļ�ԭ����)������˵������ȷ����
| A����������ʣ�࣬����Һ���ٵ�����������ֿ�ʼ�ܽ� |
| B��������ȫ���ܽ⣬����Һ�в�һ������Fe3�� |
| C��������ȫ���ܽ⣬�Ҳ���336mL����(��״��)����b=0.3 |
| D������Һ�н�������ֻ��Fe3����Cu2��ʱ����a��b�Ĺ�ϵΪ��b��80y(1��a/3) |
��һ����������ͨ��30 mLŨ��Ϊ10.00 mol/L����������Ũ��Һ�У���������ʱ�����Һ���γ�NaCl��NaClO��NaClO3������ϵ�������ж���ȷ���� (����)
| A����NaOH��Ӧ������һ��Ϊ0.3 mol |
| B��n(Na��)��n(Cl��)����Ϊ7��3 |
| C������Ӧ��ת�Ƶĵ���Ϊn mol����0.15��n��0.25 |
| D��n(NaCl)��n(NaClO)��n(NaClO3)����Ϊ11��2��1 |