��Ŀ����
��֪
��CH3COOH��CH3COONa�����ʵ��������ɵ�ϡ��Һ��pHΪ4.7��
���õ����ʵ�����HCN��NaCN��ɵ�ϡ��Һ�У�c(CN-) < c(Na+)��
������˵������ȷ����
- A.����Һ�У�c(H+)<c(OH-)
- B.����NaCN��ˮ�����ƴ���HCN�ĵ�������
- C.CH3COONa�Ĵ���������CH3COOH�ĵ���
- D.����CH3COONa��ˮ�����ƴ���CH3COOH�ĵ�������
D
������������ݢ�����Һ�����Կ�֪������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ���D��ȷ��C��ȷ�����и��ݵ���غ��֪c(CN��)��c(OH-)��c(H+)��c(Na+)������c(CN��) < c(Na+)������Һ�У�c(H+)<c(OH-)��A��ȷ��ͬ��ѡ��BҲ����ȷ�ģ���ѡD��
���㣺��������ˮ��͵���ƽ���Լ���Һ����Ե��й��ж�
�������������е��Ѷȵ����⣬���������߿����ڿ���ѧ���Ļ���֪ʶ��ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ѵ��������Ĺؼ������úõ���غ㣬����������ѧ�������Ӧ�����������ѧ����Ӧ��������
������������ݢ�����Һ�����Կ�֪������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ���D��ȷ��C��ȷ�����и��ݵ���غ��֪c(CN��)��c(OH-)��c(H+)��c(Na+)������c(CN��) < c(Na+)������Һ�У�c(H+)<c(OH-)��A��ȷ��ͬ��ѡ��BҲ����ȷ�ģ���ѡD��
���㣺��������ˮ��͵���ƽ���Լ���Һ����Ե��й��ж�
�������������е��Ѷȵ����⣬���������߿����ڿ���ѧ���Ļ���֪ʶ��ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ѵ��������Ĺؼ������úõ���غ㣬����������ѧ�������Ӧ�����������ѧ����Ӧ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
| ij�¶��£���֪CH3COOH��HClO��H2CO3��H3PO4 ����ƽ�ⳣ�����±���ʾ����0.1mol?L-1�����и���ҺpH�����ǣ������� ������������ĵ���ƽ�ⳣ��
|
 ��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��| ŨH2SO4 |
| �� |
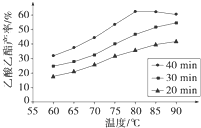
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�森������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ����1�����о�С���ʵ��Ŀ����
��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����
��3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������
��ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⣮
��֪��CO��g��+H2O��g��??H2��g��+CO2��g��ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
��1����800�淢��������Ӧ���Ա��е����ʵ���Ͷ����ݷ�Ӧ��������������Ӧ�����ƶ�����
| n��CO�� | n��H2O�� | n��H2�� | n��CO2�� | |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
C��s��+H2O��g��??CO��g��+H2��g����ƽ�ⳣ��ΪK1��
CO��g��+H2O��g��??H2��g��+CO2��g����ƽ�ⳣ��ΪK2��
��K��K1��K2֮��Ĺ�ϵ��
��3����V L�ܱ�������ͨ��10mol CO��10molˮ��������T��ﵽƽ�⣬Ȼ����ͨ����ʯ�ң������û������ȼ�գ���÷ų�������Ϊ2 842kJ����֪COȼ����Ϊ283kJ?mol-1��H2ȼ����Ϊ286kJ?mol-1������T��ƽ�ⳣ��K=