��Ŀ����
�ڸ��������������£�ijͬѧ��һ�鲿�ֱ��������ƿ���һ���ѳ�����Ĥ���������һЩС���������ã�Ȼ�����ʢˮ�ҵ�����ˮ���е������ڡ����ƿ鷴Ӧ��ȫ���������н��ռ���1.12 L��������״��������ʱ������������ȷ�Ӧǰ������0.27g��ˮ�ۺ���������Һ�������Ϊ2.0 L����Һ��NaOH��Ũ��Ϊ0.050mol/L�������ܽ������������Ӱ�죩��
(1)д����ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
(2)��ͨ������ȷ�����ƿ�����Ԫ�ص�����������
(1)д����ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
(2)��ͨ������ȷ�����ƿ�����Ԫ�ص�����������
(1)Na2O+H2O = 2NaOH��2Na+2H2O = 2NaOH+H2����2Al+2NaOH+2H2O = 2NaAlO2+3H2��
(2)89%
(2)89%

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
�����Ŀ
��Ȼ����ֲ������ڸ��������������£���������ķ������ö����γɵģ������Ȼ���������صĻ�ѧ����������ڣ�������
| A����ѧ�� | B���������� | C��̫���� | D�������� |
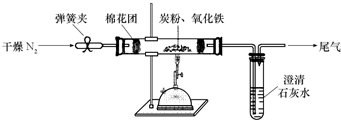


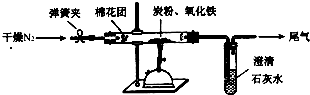 ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���
ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���