��Ŀ����
13���±������ڱ��е�һ���֣�����A��I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺| ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | |||||||
| 2 | D | E | G | |||||
| 3 | B | C | J | F | H | I |
��2��CԪ����Ԫ�����ڱ��е�λ���������ڢ�A�壮
��3������������ˮ���������ǿ����NaOH��������ǿ����HClO4�������Ե���Al��OH��3��
��4��A�ֱ���E��G��H�γɵĻ������У����ȶ���HF��
��5����B��C��E��F�У�ԭ�Ӱ뾶������Na��
��6��д��B��C����Ԫ�ص�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽAl��OH��3+NaOH=NaAlO2+2H2O��
��7��B��H����Ԫ���γɵĻ����������Ļ�ѧ������Ϊ���Ӽ�
��8��д��C�������������H���⻯��֮�䷴Ӧ�����ӷ���ʽAl2O3++6H+=2Al3++3H2O��
���� ��Ԫ�������ڱ���λ�ã���֪AΪ�⣬BΪNa��CΪAl��DΪC��EΪN��FΪP��GΪF��HΪCl��IΪAr��JΪSi��
��1��ϡ������Ļ�ѧ��������ã���Ԫ��û�������ϼۣ��������ư뵼����ϵ�Ԫ���ǹ裻
��2����C��λ�ÿ�֪��λ�ڵ������ڢ�A�壻
��3������Ԫ����Na�Ľ�������ǿ�����������Ƽ�����ǿ������������ˮ����������ǿ���Ǹ����ᣬ���������������������
��4���ǽ�����Խǿ���⻯��Խ�ȶ���
��5��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����
��6��B��C����Ԫ�ص�����������Ӧ��ˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ����ƫ��������ˮ��
��7��B��H����Ԫ���γɵĻ�����ΪNaCl��
��8��C�����������ΪAl2O3��H���⻯��ΪHCl�����߷�Ӧ�����Ȼ�����ˮ��
��� �⣺��Ԫ�������ڱ���λ�ã���֪AΪ�⣬BΪNa��CΪAl��DΪC��EΪN��FΪP��GΪF��HΪCl��IΪAr��JΪSi��
��1��ϡ������Ar�Ļ�ѧ��������ã���Ԫ��û�������ϼۣ��������ư뵼����ϵ�Ԫ���ǹ裬�ʴ�Ϊ��Ar��F��Si��
��2����C��λ�ÿ�֪��λ�ڵ������ڢ�A�壬�ʴ�Ϊ��������A��
��3������Ԫ����Na�Ľ�������ǿ����NaOH������ǿ������������ˮ����������ǿ����HClO4��Al��OH��3��������������ʴ�Ϊ��NaOH��HClO4��Al��OH��3��
��4������Ԫ����FԪ�طǽ�������ǿ���ǽ�����Խǿ���⻯��Խ�ȶ�����HF���ȶ����ʴ�Ϊ��HF��
��5��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶��������Ԫ����Na��ԭ�Ӱ뾶��ʴ�Ϊ��Na��
��6��B��C����Ԫ�ص�����������Ӧ��ˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��Al��OH��3+NaOH=NaAlO2+2H2O���ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
��7��B��H����Ԫ���γɵĻ�����ΪNaCl���������Ӽ����ʴ�Ϊ�����Ӽ���
��8��C�����������ΪAl2O3��H���⻯��ΪHCl�����߷�Ӧ�����Ȼ�����ˮ����Ӧ���ӷ���ʽΪ��Al2O3++6H+=2Al3++3H2O���ʴ�Ϊ��Al2O3++6H+=2Al3++3H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ����ض�Ԫ�������ɵĿ��飬ע���Ԫ�������ɵ��������գ�

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�| A�� | ˮ���ȵ��ܸ��¶ȶ����Էֽ� | |
| B�� | ����Һ��������������ˮ | |
| C�� | CH4��SiH4��GeH4��SnH4�۵�����Է���������������� | |
| D�� | HF��HCl��HBr��HI�����ȶ������μ��� |
| A�� | 2p | B�� | 3f | C�� | 4p | D�� | 5s |
| A�� | �����Ũ�ȴ�Сc��HX����c��HY�� | |
| B�� | ��Ũ��ʱHX��Һ�����Ա�HY��Һ�������� | |
| C�� | HX������ǿ�� | |
| D�� | ������һԪ���ϡ��100����ϡ�ͺ�����Һ��pH��Ϊ3 |
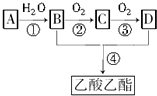
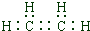 ��
�� CH3COOCH2CH3+H2O������ȡ����Ӧ��
CH3COOCH2CH3+H2O������ȡ����Ӧ�� ���˷�Ӧ����ȡ����Ӧ�����������������2.4.6-�������ױ�
���˷�Ӧ����ȡ����Ӧ�����������������2.4.6-�������ױ�