��Ŀ����
��11�֣���t��ʱ��ij ϡ��Һ�У�
ϡ��Һ�У� ��
�� ����֪
����֪ ����
����
��1�����¶��£�ˮ�����ӻ�����Kw=__________________��
��2���ڸ��¶��£��� ��ϡ������
��ϡ������ ��
�� ��Һ��Ϻ���Һ��pH=_________����ʱ����Һ����ˮ�����
��Һ��Ϻ���Һ��pH=_________����ʱ����Һ����ˮ����� =_________��
=_________��
��3�����¶��£��� ���
��� ��ijǿ����Һ��1���
��ijǿ����Һ��1��� ��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���
��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ��� ��ǿ���
��ǿ���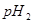 ֮��Ӧ����Ĺ�ϵ��_________��
֮��Ӧ����Ĺ�ϵ��_________��
��4�����¶��£�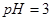 ��������Һ��
��������Һ�� ��
�� ��Һ�������Ϻ����Һ��
��Һ�������Ϻ����Һ�� _________����>7��=7��<7����
_________����>7��=7��<7����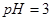 �Ĵ�����Һ��
�Ĵ�����Һ�� ��
�� ��Һ�������Ϻ����Һ��
��Һ�������Ϻ����Һ�� _________����>7��=7��<7����
_________����>7��=7��<7����
 ϡ��Һ�У�
ϡ��Һ�У� ��
�� ����֪
����֪ ����
������1�����¶��£�ˮ�����ӻ�����Kw=__________________��
��2���ڸ��¶��£���
 ��ϡ������
��ϡ������ ��
�� ��Һ��Ϻ���Һ��pH=_________����ʱ����Һ����ˮ�����
��Һ��Ϻ���Һ��pH=_________����ʱ����Һ����ˮ����� =_________��
=_________����3�����¶��£���
 ���
��� ��ijǿ����Һ��1���
��ijǿ����Һ��1��� ��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���
��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ��� ��ǿ���
��ǿ���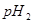 ֮��Ӧ����Ĺ�ϵ��_________��
֮��Ӧ����Ĺ�ϵ��_________����4�����¶��£�
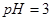 ��������Һ��
��������Һ�� ��
�� ��Һ�������Ϻ����Һ��
��Һ�������Ϻ����Һ�� _________����>7��=7��<7����
_________����>7��=7��<7����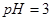 �Ĵ�����Һ��
�Ĵ�����Һ�� ��
�� ��Һ�������Ϻ����Һ��
��Һ�������Ϻ����Һ�� _________����>7��=7��<7����
_________����>7��=7��<7������11�֣���һ����1�֣�����ÿ��2�֣���1��
��2��11
��3�� ����
���� ��
��
��4��<7��<7

��2��11

��3��
 ����
���� ��
����4��<7��<7
��1��Kw=c(H+)c(OH-)=10-a��10-b=10-(a+b)=10-12��2����������кͣ�NaOH������c(OH-)==0.1mol/L��pH=-lg=11��ˮ�����������Ϊ10-11mol/L����3��n(H+)=n(OH-)��100��10-a=1��10-(12-b)��a+b=14����4��pH=3��pH=9���������Ӻ�����������Ũ����ȣ��кͺ�pH=6����������������кͣ�������������ԣ�pH<6��

��ϰ��ϵ�д�
�����Ŀ
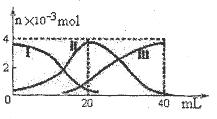
 2H�� + S2-
2H�� + S2- CH3COO����H���У�Ҫʹ����ƽ��������c(H��)����Ӧ��ȡ�Ĵ�ʩ��(����)
CH3COO����H���У�Ҫʹ����ƽ��������c(H��)����Ӧ��ȡ�Ĵ�ʩ��(����) H����CH3COO���������������й���������ʹƽ�������ƶ�����
H����CH3COO���������������й���������ʹƽ�������ƶ�����