��Ŀ����
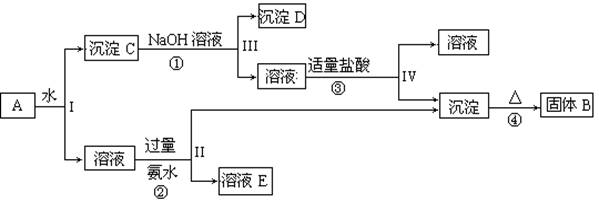
��13�֣�ij�����A����KAl(SO4)2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��C��D��E�������ʵĻ�ѧʽ
����B ������C ������D ����ҺE ��
��3��д���١��ڡ��ۡ����ĸ���Ӧ����ʽ���ڡ���д���ӷ���ʽ�١���д��ѧ����ʽ��
�� ���� ��
�� ���� ��
��13�֣�
��1������ ��1�֣�
��2��Al2O3��Al2O3��Fe2O3�� Fe2O3��K2SO4 ��(NH4)2SO4 ��NH3?H2O��ÿ��1�֣�
��3����Al2O3 + 2 NaOH +3H2O =" 2" Na [Al(OH)4]��2�֣�
��Al3+��3NH3?H2O��Al(OH) 3����3NH4+��2�֣�
��[Al(OH)4]-��H+��Al(OH) 3����H2O ��2�֣�
��2Al(OH) 3 Al2O3��3H2O ��2�֣�
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
 �ݴ˻ش��������⣺
�ݴ˻ش��������⣺