��Ŀ����
9��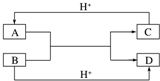 ��1����֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮�������ͼ��ʾ��ת����ϵ����Ӧ�����Ѿ���ȥ����
��1����֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮�������ͼ��ʾ��ת����ϵ����Ӧ�����Ѿ���ȥ���������A��B��C��D����10���ӵ����ӣ���д����A����ʽ��
 ��D�Ľṹʽ��H-O-H��
��D�Ľṹʽ��H-O-H�������A��C��18���ӵ����ӣ�B��D��10���ӵ����ӣ���д����A��B����Һ�з�Ӧ�����ӷ���ʽΪHS-+OH-�TS2-+H2O�� �����������ӷ���ʽ�������ж�C��B������ӵ�������С��OH-��S2-�����û�ѧʽ�����ӷ��ű�ʾ����
��2����һ10�������ʣ���ͬ�����¶�H2������ܶ�Ϊ8�����³�ѹ�£�3.2g�������������������ȼ�պ�ų�akJ��������a��0����д��������ȼ���ȵ��Ȼ�ѧ����ʽ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-5akJ•mol-1��
��3.2g������ȼ�պ�IJ���ͨ��100mL3mol•L-1��NaOH��Һ�У���ַ�Ӧ�����õ���Һ������Ũ�ȴ�С��ϵΪ��Na+��HCO3-��CO32-��OH-��H+��
���� ��1����10������A��B��Ӧ�õ�����10��������Ӧ��笠����������������ӷ�Ӧ�õ�������ˮ����C��B�����������ӷ�Ӧ������֪AΪNH4+��BΪOH-��CΪNH3��DΪH2O��
�����A��C����18���ӵ����ӣ�B��D��10�������ӣ����ת����ϵ�����ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��
��2����һ10�������ʣ���ͬ�����¶�H2������ܶ�Ϊ8�����������Է�������Ϊ16���������ΪCH4������1mol����ȼ�շų���������ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
3.2g�������ʵ���Ϊ0.2mol����ȼ�����ɶ�����̼Ϊ0.2mol��NaOH���ʵ���Ϊ0.3mol������1��2��n��CO2����n��NaOH��=2��3��1��1��������NaHCO3��Na2CO32�������������غ㡢̼ԭ���غ����������ʵ����������Һ��HCO3-��CO32-ˮ������жϣ�
��� �⣺��1����10������A��B��Ӧ�õ�����10��������Ӧ��笠����������������ӷ�Ӧ�õ�������ˮ����C��B�����������ӷ�Ӧ������֪AΪNH4+��BΪOH-��CΪNH3��DΪH2O��NH4+�ĵ���ʽΪ ��H2O�ṹʽΪH-O-H��
��H2O�ṹʽΪH-O-H��
�ʴ�Ϊ�� ��H-O-H��
��H-O-H��
�����A��C����18���ӵ����ӣ�B��D��10�������ӣ����ת����ϵ�����ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��A��B����Һ�з�Ӧ�����ӷ���ʽΪ��HS-+OH-�TS2-+H2O���������ӷ���ʽ�������жϽ�����ӵ�������С��OH-��S2-��
�ʴ�Ϊ��HS-+OH-�TS2-+H2O��OH-��S2-��
��2����һ10�������ʣ���ͬ�����¶�H2������ܶ�Ϊ8�����������Է�������Ϊ16���������ΪCH4��1mol����ȼ�շų�������ΪakJ��$\frac{1mol��16g/mol}{3.2g}$=5a kJ����Ӧ����д�Ȼ�ѧ����ʽ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-5akJ•mol-1��
3.2g�������ʵ���Ϊ$\frac{3.2g}{16g/mol}$=0.2mol����ȼ�����ɶ�����̼Ϊ0.2mol��NaOH���ʵ���Ϊ0.1L��3mol/L=0.3mol������1��2��n��CO2����n��NaOH��=2��3��1��1��������NaHCO3��Na2CO32����������ʵ����ֱ�Ϊxmol��ymol�������������غ㡢̼ԭ���غ�ɵã�$\left\{\begin{array}{l}{x+y=0.2}\\{x+2y=0.3}\end{array}\right.$�����x=y=0.1�������Һ��HCO3-��CO32-ˮ��ʼ��ԣ���̼�������ˮ��̶ȴ���̼������ģ�����Һ������Ũ�ȴ�СΪ��Na+��HCO3-��CO32-��OH-��H+��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-5akJ•mol-1�� Na+��HCO3-��CO32-��OH-��H+��
���� ���⿼�������ƶϣ��������ճ���10���ӡ�18�������Ľṹ������Ӧ�ã��Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ����������Һʱ����Ũ��������ע��ʢ��ˮ������ƿ�н������� | |
| B�� | ��������ƽ����8.75g NaCl���� | |
| C�� | �����Һ�������ʱ��Ӧʹ�¶ȼ�ˮ�����û�ڻ��Һ�� | |
| D�� | ��Һʱ�����ϲ�Һ��ӷ�Һ©���Ͽڵ������²�Һ��ӷ�Һ©���¿ڷų� |
| A�� | ɽ������ | B�� | �����ɸ� | C�� | ľ�ѳ��� | D�� | �������� |
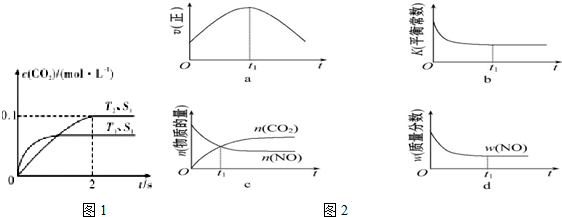
 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��2��ͼ�б�ʾNO2�ı仯��������b����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=1.5��10-3mol•L-1•s-1��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����be��
a��v��NO2��=2v��O2�� b��������ѹǿ���ֲ���
c��NO��O2��NO2��Ũ��֮��Ϊ2��1��2 d���������ܶȱ��ֲ��� e���������������ɫ���ٱ仯
��4����ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ��Ĵ�ʩ������Ӧ���Ũ�ȣ�
| A�� | ͭ��ϡ����ķ�Ӧ��Cu2++4H++2NO3-�TCu2++2NO2��+2H2O | |
| B�� | ������ˮ��Ӧ��Fe3++Cu�TFe2++Cu2+ | |
| C�� | �Ȼ�����Һ���백ˮ��Al3++3OH-�TAl��OH��3�� | |
| D�� | ʵ������������MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O |
| ʵ����� | �� | �� | �� |
| �Ͻ�����/mg | 510 | 770 | 918 |
| �������/mL | 560 | 672 | 672 |
| A�� | ����������ʵ���У�������ǹ����� | |
| B�� | ��������ʵ���Ũ��Ϊ1.0mol•L-1 | |
| C�� | �Ͻ���þ�������ʵ���֮��Ϊ1��1 | |
| D�� | �������������ʵ���Ϊ0.018mol |