��Ŀ����
��֪X��Y��Z��W����Ԫ�طֱ���Ԫ�����ڱ������������ڵ�Ԫ�أ���ԭ��������������X��Wͬ���壬Y��ZΪͬ���ڵ�����Ԫ�ء�Wԭ�ӵ�����������Y��Zԭ������������֮�͡�Y���⻯���������3�����ۼ���Zԭ�������������Ǵ�����������3�������ƶϣ�
��1��X��Z����Ԫ�ص�Ԫ�ط��ţ�X_________��Z__________��
(2)������Ԫ���������γɵĻ������У�����ˮ�Լ��Ե���̬�⻯��ĽṹʽΪ�� �����Ĺ��ۼ����� ������ԡ��Ǽ��ԣ������������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ ��
(3)��X��Y��Z���γɵij������ӻ�������___________���û�������W������������ˮ�����Ũ��Һ����ʱ��Ӧ�����ӷ���ʽΪ��___________________ ______ ��
X��W�γɵĻ�������ˮ��Ӧʱ��ˮ������ �������������ԭ������
(1) H O
(2) �� ����ʽ ��
(3)NH4NO3 NH4++OH-=NH3+H2O ������
����:��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ








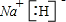
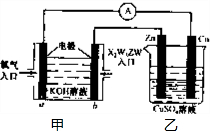 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��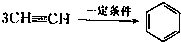 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��