��Ŀ����
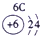
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�һ���ڱ��е�λ�ã��ش���������

��1���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����________________��д��ѧʽ����
��2���ɱ���Ԫ�ؿ�����ɶ���Ư������д���������ֳ���Ư���Ļ�ѧʽ��_________��_______��
��3���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ�____________________��
��4���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��
_________________________��
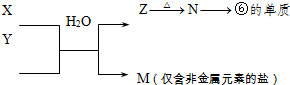
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��W�ɷ������·�Ӧ��
��2���ɱ���Ԫ�ؿ�����ɶ���Ư������д���������ֳ���Ư���Ļ�ѧʽ��_________��_______��
��3���١��ܡ�������Ԫ���γɵĻ������л�ѧ�������ͣ�____________________��
��4���ں͢��γɵĻ�������ܺ͢��γɵĻ�����֮�䷢��������ԭ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��
_________________________��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��W�ɷ������·�Ӧ��

��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
����֪MΪ�����Σ���M��Һ�и�����Ũ���ɴ�С����˳��Ϊ��
c��_____��>c��_____��>c��_____��>c��_____�����������������ӷ��ţ���
����֪MΪ�����Σ���M��Һ�и�����Ũ���ɴ�С����˳��Ϊ��
c��_____��>c��_____��>c��_____��>c��_____�����������������ӷ��ţ���
��1��HNO3>H2CO3>H2SiO3
��2��NaClO��HClO����H2O2��SO2��Na2O2��H2C2O4�Ⱥ������ɣ�
��3�����Ӽ������ۼ������Թ��ۼ���
��4��2Na2O2+2CO2==2Na2CO3+O2
��5����Al3+3NH3��H2O==Al(OH)3��+3NH4+����c��NO3-��>c��NH4+��>c��H+��>c��OH-��
��2��NaClO��HClO����H2O2��SO2��Na2O2��H2C2O4�Ⱥ������ɣ�
��3�����Ӽ������ۼ������Թ��ۼ���
��4��2Na2O2+2CO2==2Na2CO3+O2
��5����Al3+3NH3��H2O==Al(OH)3��+3NH4+����c��NO3-��>c��NH4+��>c��H+��>c��OH-��

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ








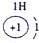
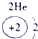
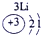
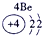
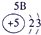
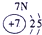
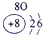
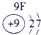
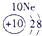
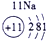





 ��ʾ����
��ʾ����