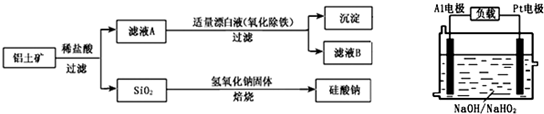
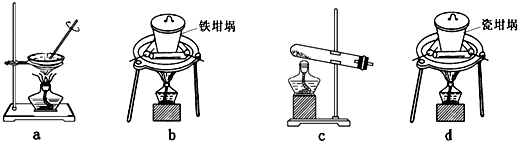
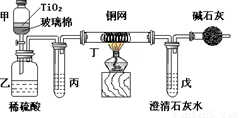
��Ŀ����
�����1����Ƶĺ���
�����Ӧ��___________��ijЩ�����������һ��������������Ͻ�Ĺ��̡�
��2����Ƶ�Ŀ��
��Ƶ�Ŀ����Ҫ��___________________��
��3����Ƶ�ԭ��
������_________________��
������_________________��
���Һ��______________________��
��4��ͭ�ľ���
��װ��Ҫ��
������___________��������___________���������Һ��______________________��
�ڻ�ѧԭ��
������Ӧ��Cu-2e-![]() Cu2+
Cu2+
Zn-2e-![]() Zn2+
Zn2+
������Ӧ��__________________
�۵���ص�
a.��ͭ�е�ͭ��Ǩ�ơ�����ͭ�ϣ�
b.CuSO4��Һ��Ũ��_________��
��1�����ԭ��
��2��ʹ������ǿ����ʴ�������������ۺͱ���Ӳ��
��3���Ʋ���� �Ƽ� ���жƲ�����ĵ������Һ
��4���ٴ�ͭ ��ͭ CuSO4��Һ
��Cu2++2e-![]() Cu
Cu
�ۻ�������

��12�֣�����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֡�K2Cr2O7��CrO3�����������ӡȾ�����ϡ���Ƶȹ�ҵ�У��ǹ�ҵ����ɸ���Ⱦ����Ҫԭ���ڱ���ġ������ҡ��¼��У�������Ϊ�ù�ҵƤ����½��ϻ���ƤЬ��Ϊԭ���ƳɵĹ�ҵ������ð���ʳ�������Ƴɽ��ң���ɽ����ڵĸ����س��ꡣ
��1��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ�� 
��A ��ʱʣ�����ijɷ��� ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ ��
��2��Cr(��)��Ҫ��CrO42����Cr2O72����̬���ڣ������������¾��к�ǿ�������ԣ���������Һ�д�������ת����CrO42��(��ɫ)+2H+ Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ�� ��
| A����NaOH | B�������� | C�������� | D����AgNO3 |
�ٵ������� NaCl �������� ��
��д�������ĵ缫��Ӧʽ ��
��д��Fe2����Cr2O72��������Ӧ����Fe3����Cr3�������ӷ�Ӧ����ʽ ��
����֪�������Һ��c(Fe3��)=2.0��10��13 mol��L��1������Һ��c(Cr3��)Ϊ mol��L��1��