ЬтФПФкШн
ЁОЬтФПЁПЯжгУ0.1000 molЁЄLЃ1KMnO4ЫсадШмвКЕЮЖЈЮДжЊХЈЖШЕФЮоЩЋH2C2O4ШмвКЃЌЗДгІРызгЗНГЬЪНЪЧЃК2MnO4ЃЃЋ5H2C2O4ЃЋ6H+ = 2Mn2+ЃЋ10CO2ЁќЃЋ8H2O
ЬюПеЭъГЩЮЪЬтЃК
ЃЈ1ЃЉИУЕЮЖЈЪЕбщЫљашЕФВЃСЇвЧЦїга______________ЁЃЃЈЬюзжФИЃЉ
AЃЎЫсЪНЕЮЖЈЙмBЃЎМюЪНЕЮЖЈЙм CЃЎСПЭВ DЃЎзЖаЮЦП EЃЎЬњМмЬЈFЃЎЕЮЖЈЙмМаGЃЎЩеБHЃЎАзжН IЃЎТЉЖЗ
ЃЈ2ЃЉВЛгУ________(ЬюЁАЫсЁБЛђЁАМюЁБ)ЪНЕЮЖЈЙмЪЂЗХИпУЬЫсМиШмвКЁЃЪдЗжЮідвђ___________________________________________ЁЃ
ЃЈ3ЃЉЕЮЖЈжеЕуЕФЯжЯѓЮЊ___________________________________ЁЃ
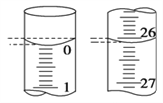
ЃЈ4ЃЉШєЕЮЖЈПЊЪМКЭНсЪјЪБЃЌЕЮЖЈЙмжаЕФвКУцШчЭМЫљЪОЃЌдђЦ№ЪМЖСЪ§ЮЊ________mLЃЌжеЕуЖСЪ§ЮЊ________mLЁЃ
ЃЈ5ЃЉФГбЇЩњИљОн3ДЮЪЕбщЗжБ№МЧТМгаЙиЪ§ОнШчЯТБэЃК
ЕЮЖЈ ДЮЪ§ | Д§ВтH2C2O4ШмвКЕФЬхЛ§/mL | 0.1000 mol/L KMnO4ЕФЬхЛ§ЃЈmLЃЉ | ||
ЕЮЖЈЧАПЬЖШ | ЕЮЖЈКѓПЬЖШ | ШмвКЬхЛ§/mL | ||
ЕквЛДЮ | 25.00 | 0.00 | 26.11 | 26.11 |
ЕкЖўДЮ | 25.00 | 1.56 | 30.30 | 28.74 |
ЕкШ§ДЮ | 25.00 | 0.22 | 26.31 | 26.09 |
вРОнЩЯБэЪ§ОнСаЪНМЦЫуИУH2C2O4ШмвКЕФЮяжЪЕФСПХЈЖШЮЊ_______________ЁЃ
ЃЈ6ЃЉЯТСаВйзїжаПЩФмЪЙВтЖЈНсЙћЦЋЕЭЕФЪЧ___________(ЬюзжФИ)ЁЃ
AЃЎЫсЪНЕЮЖЈЙмЮДгУБъзМвКШѓЯДОЭжБНгзЂШыKMnO4БъзМвК
BЃЎЕЮЖЈЧАЪЂЗХВнЫсШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдя
CЃЎЫсЪНЕЮЖЈЙмМтзьВПЗждкЕЮЖЈЧАУЛгаЦјХнЃЌЕЮЖЈКѓгаЦјХн
DЃЎЖСШЁKMnO4БъзМвКЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§
ЁОД№АИЁП ADG Мю ИпУЬЫсМиОпгаЧПбѕЛЏадФмИЏЪДЯ№НКЙмЃЈЛђИпУЬЫсМиШмвКФмАбЯ№НКЙмбѕЛЏЃЉ ЕЮШызюКѓвЛЕЮИпУЬЫсМиШмвКЪБЃЌШмвКДгЮоЩЋБфЮЊЧГзЯЩЋЃЌЧвАыЗжжгФкВЛЛжИДдЩЋ 0.00 26.10 0.2610 mol/L CD
ЁОНтЮіЁПЃЈ1ЃЉИУбѕЛЏЛЙдЕЮЖЈЫљашВЃСЇвЧЦїгаЃКЪЂзАЫсадKMnO4БъзМвККЭШЁгУД§ВтвКH2C2O4ШмвКЕФЫсЪНЕЮЖЈЙмЁЂЪЂзАД§ВтвКH2C2O4ШмвКЕФзЖаЮЦПЁЂЕїНквКУцМАИЯЦјХнЪБЪЂНгШмвКгУЕФЩеБЃЌЫљвдгІбЁADGЁЃ
ЃЈ2ЃЉвђЮЊИпУЬЫсМиОпгаЧПбѕЛЏадФмИЏЪДЯ№НКЙмЃЌЫљвдВЛгУМюЪНЕЮЖЈЙмЪЂЗХИпУЬЫсМиШмвКЁЃ
ЃЈ3ЃЉMnO4-ЮЊзЯЩЋЃЌK+ЮЊЮоЩЋЃЌЕБЕЮШызюКѓвЛЕЮИпУЬЫсМиШмвКЪБЃЌШмвКДгЮоЩЋБфЮЊЧГзЯЩЋЃЌЧвАыЗжжгФкВЛЛжИДдЩЋЃЌдђЫЕУїДяЕНЕЮЖЈжеЕуЁЃ
ЃЈ4ЃЉШчЭМЫљЪОЃЌЖСЪ§ЪБЪгЯпгІгыАМвКУцзюЕЭДІЯрЧаЃЌЙЪЦ№ЪМЖСЪ§ЮЊ0.00mLЃЌжеЕуЖСЪ§ЮЊ26.10mLЁЃ
ЃЈ5ЃЉЕк2зщЪ§ОнгыСэЭтСНзщВюБ№НЯДѓЃЌИљОнЪ§ОнЕФгааЇадЃЌгІЩсШЅЕк2зщЪ§ОнЃЌЯћКФЕФV(KMnO4)=(26.11+26.09)mLЁТ2=26.10mLЃЌгЩЗНГЬЪН2MnO4ЃЃЋ5H2C2O4ЃЋ6H+ = 2Mn2+ЃЋ10CO2ЁќЃЋ8H2OПЩЕУЃЌn(H2C2O4)= ![]() n(MnO4-)ЃЌМДЃКc(H2C2O4)ЁС0.025L=
n(MnO4-)ЃЌМДЃКc(H2C2O4)ЁС0.025L=![]() ЁС0.1000molL-1ЁС0.02610LЃЌНтЕУc(H2C2O4)=0.2610molL-1ЁЃ
ЁС0.1000molL-1ЁС0.02610LЃЌНтЕУc(H2C2O4)=0.2610molL-1ЁЃ
ЃЈ6ЃЉИљОнc(Д§)= 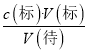 НјааЮѓВюЗжЮіЃЌAЯюЃЌЫсЪНЕЮЖЈЙмЮДгУБъзМвКШѓЯДОЭжБНгзЂШыKMnO4БъзМвКЃЌдђБъзМвКЕФХЈЖШЦЋаЁЃЌдьГЩV(Бъ)ЦЋДѓЃЌc(Д§)ЦЋДѓЃЌЙЪAДэЮѓЃЛBЯюЃЌЕЮЖЈЧАЪЂЗХH2C2O4ШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдяЃЌЖдV(Бъ)УЛгагАЯьЃЌc(Д§)ВЛБфЃЌЙЪBДэЮѓЃЛCЯюЃЌЫсЪНЕЮЖЈЙмМтзьВПЗждкЕЮЖЈЧАУЛгаЦјХнЃЌЕЮЖЈКѓгаЦјХнЃЌдьГЩV(Бъ)ЦЋаЁЃЌc(Д§)ЦЋаЁЃЌЙЪCе§ШЗЃЛDЯюЃЌЖСШЁKMnO4БъзМвКЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§ЃЌдьГЩV(Бъ)ЦЋаЁЃЌc(Д§)ЦЋаЁЃЌЙЪDе§ШЗЁЃ
НјааЮѓВюЗжЮіЃЌAЯюЃЌЫсЪНЕЮЖЈЙмЮДгУБъзМвКШѓЯДОЭжБНгзЂШыKMnO4БъзМвКЃЌдђБъзМвКЕФХЈЖШЦЋаЁЃЌдьГЩV(Бъ)ЦЋДѓЃЌc(Д§)ЦЋДѓЃЌЙЪAДэЮѓЃЛBЯюЃЌЕЮЖЈЧАЪЂЗХH2C2O4ШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдяЃЌЖдV(Бъ)УЛгагАЯьЃЌc(Д§)ВЛБфЃЌЙЪBДэЮѓЃЛCЯюЃЌЫсЪНЕЮЖЈЙмМтзьВПЗждкЕЮЖЈЧАУЛгаЦјХнЃЌЕЮЖЈКѓгаЦјХнЃЌдьГЩV(Бъ)ЦЋаЁЃЌc(Д§)ЦЋаЁЃЌЙЪCе§ШЗЃЛDЯюЃЌЖСШЁKMnO4БъзМвКЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§ЃЌдьГЩV(Бъ)ЦЋаЁЃЌc(Д§)ЦЋаЁЃЌЙЪDе§ШЗЁЃ

 РшУїЮФЛЏКЎМйзївЕЯЕСаД№АИ
РшУїЮФЛЏКЎМйзївЕЯЕСаД№АИ КЎМйЬьЕижиЧьГіАцЩчЯЕСаД№АИ
КЎМйЬьЕижиЧьГіАцЩчЯЕСаД№АИЁОЬтФПЁПзщГЩЩњУќЕФзюЛљБОдЊЫижЎвЛЪЧЬМЃЌЦфЕЅжЪМАЛЏКЯЮядкбаОПКЭЩњВњжагааэЖрживЊгУЭОЁЃЧыЛиД№ЯТСаЮЪЬт:
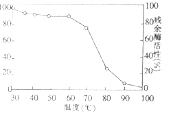
ЃЈ1ЃЉЛљЬЌЬМдзгМлЕчзгдзгЙьЕРБэЪОЪНЮЊ________ЃЌЦфКЫЭтга________жжПеМфдЫЖЏзДЬЌЕФЕчзг.
ЃЈ2ЃЉ гаЛњЮяжаЖМКЌгаЬМдзгЃЌетКЭЬМдзгЕчзгдЦЕФГЩМќЬиЕугаЙиЃЌЪдНтЪЭгаЛњЮяжжРрЗБЖрЕФдвђ________ ЁЃ
ЃЈ3ЃЉ БШНЯЯТСаЬМЫсбЮдквЛЖЈЮТЖШЯТЛсЗЂЩњЗжНтЕФЮТЖШКЭЖдгІЕФбєРызгАыОЖЃЌЗжЮіЦфБфЛЏЙцТЩМАдвђ_____________ЁЃ
ЬМЫсбЮ | MgCO3 | CaCO3 | BaCO3 | SrCO3 |
ШШЗжНтЮТЖШ/Ёц | 402 | 900 | 1172 | 1360 |
бєРыСЫАыОЖ/pm | 66 | 99 | 112 | 135 |
ЃЈ4ЃЉ1828 ФъЃЌЕТЙњЛЏбЇМвЮкРе(FЁЄWohler)ГхЦЦСЫЩњУќСІбЇЫЕЕФЪјИПЃЌдкЪЕбщЪвРяНЋЮоЛњЮяЧшЫсяЇ(NH4CNO)ШмвКеєЗЂЃЌЕУЕНСЫгаЛњЮяФђЫи[CO(NH2)2]ЁЃФђЫижаCЁЂNЁЂOЕквЛЕчРыФмДѓаЁЫГађЮЊ____________ЁЃ
ЃЈ5ЃЉ ЬМЕФЭЌЫивьаЮЬхгаЖржжЃЌЦфжавЛжжЮЊЪЏФЋЃЌЯЕЦНУцВузДНсЙЙЁЃЭЌвЛВуФкУПИіЬМдзггыЦфЫќШ§ИіЬМдзгвдC -C МќЯрСЌЙЙГЩЦНУце§СљБпаЮЃЌЧвВуМфПЩвдЛЌЖЏЃЌЫќЕФНсЙЙШчЭМЫљЪОЁЃЦфжаЬМдзгЕФдгЛЏЗНЪНЮЊ_______ЃЌВуМфДцдкЕФзїгУСІЮЊ__________ЁЃ
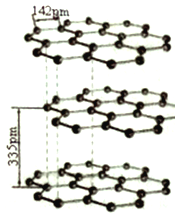
ЃЈ6ЃЉЪЏФЋОЇЬхЖўЮЌНсЙЙЕФЬиЪтадЁЃ
ЂйЭЌвЛВуУцФкЕФЦНУцСљдЊВЂЛЗНсЙЙ(МћЩЯЭМ)ЁЃдкКЌlmolCдзгЕФЪЏФЋжаЃЌга_____ИіЦНУце§СљБпаЮЁЃ
ЂквбжЊЪЏФЋВуМфОрЮЊ335pmЃЌC-CМќГЄЮЊ142pmЃЌЦфУмЖШ______g/cm3(вбжЊЃКlpm=10-10cmЁЃСаГіМЦЫуЪНМДПЩ)