��Ŀ����
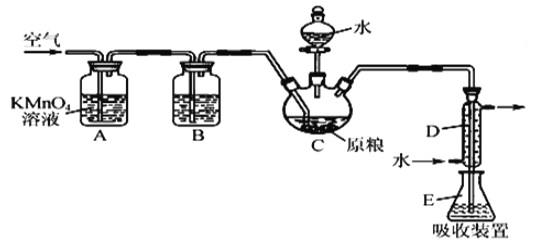
����Ŀ��A��B��C��D��E��ԭ���������ε��������ֳ���Ԫ�ء�A��BԪ����ɵ���̬������M��ˮ��Һ�ʼ��ԣ�CԪ���ǵؿ��к�������Ԫ�أ�D�ĵ�����C�ĵ�����ȼ�պ�IJ������ʹƷ����Һ��ɫ��E�ǽ���Ԫ�ء�
(1)д��A��C����Ԫ����ɵĻ�����A2C2�ĵ���ʽ_______��
(2)����E����Ͷ�������У�������dz��ɫ��Һ�Ρ���ε�������Һ��A2C2��Ӧ�����ӷ���ʽΪ______________________________________��
(3)R��B�������ͨ������³ʺ���ɫ������һ�Թ�R����ʹԪ��Bȫ��ת��Ϊ��������������Ӧ��ˮ����ʽ�������ʵ���������ʢ��R���Թܵ�����ˮ���У�______��
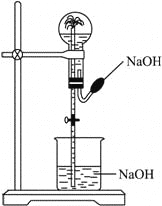
(4)��������Ѱ����ʵĴ����͵缫���ϣ���A2��B2Ϊ�缫��Ӧ���HCl��NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ���д���õ�ص�������Ӧʽ_____________���ŵ�ʱ����Һ�е�H+����_________(��������)��
���𰸡�![]() 2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ���Թ��л���ͨ���������� N2 +8H+ +6e- =2NH
2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ���Թ��л���ͨ���������� N2 +8H+ +6e- =2NH![]() ����
����
��������
A��B��C��D��E��ԭ���������ε��������ֳ���Ԫ�ء�CԪ���ǵؿ��к�������Ԫ�أ���CΪOԪ�أ�A��B��ɵ���̬������M��ˮ��Һ�ʼ��ԣ�����֪BΪNԪ�ء�AΪHԪ�أ�MΪNH3��D�ĵ�����O2��ȼ�ղ����ʹƷ����Һ��ɫ������֪DΪSԪ�ء�
(1) A��C����Ԫ����ɵĻ�����A2C2ΪH2O2�����ڹ��ۻ���������ʽΪ![]() ��
��
(2) E�ǽ���Ԫ�أ���E����Ͷ�뵽������Һ�У�������dz��ɫ��ҺN����EΪFeԪ�أ�NΪFeCl2��Fe2+���л�ԭ�ԣ�H2O2���������ԣ��ʶ��߷�Ӧ�����ӷ���ʽΪ2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O��
(3)R��B�������ͨ������³ʺ���ɫ����RΪNO2������NO2ȫ��ת��ΪHNO3����ʢ��NO2���Թܵ�����ˮ���л������Թ��л���ͨ������������
(4) ��H2��N2Ϊ�缫��Ӧ���HCl��NH4Cl��ҺΪ�������Һ��������ȼ�ϵ�أ�����H2��N2�ϳɰ�ԭ����֪��H2ʧ���ӣ��Ӹ���ͨ�룬N2�õ��ӣ�������ͨ�룬��������ӦʽΪN2 +8H+ +6e- =2NH![]() ���ŵ�ʱ����Һ�е�H+Ϊ�����ӣ�Ӧ����������
���ŵ�ʱ����Һ�е�H+Ϊ�����ӣ�Ӧ����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�