��Ŀ����
��1���ڱ�״���£�1.7g������ռ�����Ϊ______L�������״����______L���⺬����ͬ��Ŀ����ԭ�ӣ�
��2��ij��̬������Ļ�ѧʽΪRO2���ڱ�״���£�1.28g������������Ϊ448mL������������Ħ������Ϊ______��R�����ԭ������Ϊ______��
��3����֪CO��CO2�Ļ������������16.0g����״�������Ϊ8.96L�������֪�û�������к�CO______g������CO2�ڱ�״���µ����Ϊ______L��
��4��ͬ��ͬѹ�£�SO2�뺤�����ܶ�֮��Ϊ______����������ͬ����������������Ϊ______��
�⣺��1��1.7g���������ʵ���Ϊ =0.1mol�������Ϊ0.1mol��22.4L/mol=2.24L��
=0.1mol�������Ϊ0.1mol��22.4L/mol=2.24L��
�������״����VL���⺬����ͬ��Ŀ����ԭ�ӣ���
0.1mol��3= ��2�����V=3.36���ʴ�Ϊ��2.24��3.36��
��2�����V=3.36���ʴ�Ϊ��2.24��3.36��
��2����������ʵ���Ϊ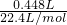 =0.02mol�����������Ħ������Ϊ
=0.02mol�����������Ħ������Ϊ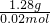 =64g?mol-1��
=64g?mol-1��
�������Ħ����������Է�����������ֵ����ȣ�R�����ԭ������Ϊ64-16��2=32��
�ʴ�Ϊ��64g?mol-1��32��
��3����״�������Ϊ8.96L��������������ʵ���Ϊ =0.4mol��
=0.4mol��
��CO��CO2�����ʵ����ֱ�Ϊx��y����
 �����x=0.1mol��y=0.3mol��
�����x=0.1mol��y=0.3mol��
CO������Ϊ0.1mol��28g/mol=2.80g��CO2�ڱ�״���µ����Ϊ0.3mol��22.4L/mol=6.72L��
�ʴ�Ϊ��2.80��6.72��
��4��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ���SO2�뺤�����ܶ�֮��Ϊ64g/mol��4g/mol=16��1��
��ͬ����ʱ�������Ħ�������ɷ��ȣ���������ͬ����������������Ϊ4g/mol��64g/mol=1��16��
�ʴ�Ϊ��16��1��1��16��
��������1������n= ��V=n��Vm�����ӵĹ��������㣻
��V=n��Vm�����ӵĹ��������㣻
��2������n= ��V=n��Vm�������Ħ����������Է�����������ֵ�������������
��V=n��Vm�������Ħ����������Է�����������ֵ�������������
��3������V=n��Vm�����û����������������ʵ�������ʽ���㣻
��4��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ���ͬ����ʱ�������Ħ�������ɷ��ȣ�
���������⿼�������ʵ���Ϊ���ĵ��йؼ��㣬��ȷ��������������ʵ���������٤�����ɼ��������ǽ����Ĺؼ���
 =0.1mol�������Ϊ0.1mol��22.4L/mol=2.24L��
=0.1mol�������Ϊ0.1mol��22.4L/mol=2.24L���������״����VL���⺬����ͬ��Ŀ����ԭ�ӣ���
0.1mol��3=
 ��2�����V=3.36���ʴ�Ϊ��2.24��3.36��
��2�����V=3.36���ʴ�Ϊ��2.24��3.36����2����������ʵ���Ϊ
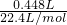 =0.02mol�����������Ħ������Ϊ
=0.02mol�����������Ħ������Ϊ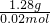 =64g?mol-1��
=64g?mol-1���������Ħ����������Է�����������ֵ����ȣ�R�����ԭ������Ϊ64-16��2=32��
�ʴ�Ϊ��64g?mol-1��32��
��3����״�������Ϊ8.96L��������������ʵ���Ϊ
 =0.4mol��
=0.4mol����CO��CO2�����ʵ����ֱ�Ϊx��y����
 �����x=0.1mol��y=0.3mol��
�����x=0.1mol��y=0.3mol��CO������Ϊ0.1mol��28g/mol=2.80g��CO2�ڱ�״���µ����Ϊ0.3mol��22.4L/mol=6.72L��
�ʴ�Ϊ��2.80��6.72��
��4��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ���SO2�뺤�����ܶ�֮��Ϊ64g/mol��4g/mol=16��1��
��ͬ����ʱ�������Ħ�������ɷ��ȣ���������ͬ����������������Ϊ4g/mol��64g/mol=1��16��
�ʴ�Ϊ��16��1��1��16��
��������1������n=
 ��V=n��Vm�����ӵĹ��������㣻
��V=n��Vm�����ӵĹ��������㣻��2������n=
 ��V=n��Vm�������Ħ����������Է�����������ֵ�������������
��V=n��Vm�������Ħ����������Է�����������ֵ���������������3������V=n��Vm�����û����������������ʵ�������ʽ���㣻
��4��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ���ͬ����ʱ�������Ħ�������ɷ��ȣ�
���������⿼�������ʵ���Ϊ���ĵ��йؼ��㣬��ȷ��������������ʵ���������٤�����ɼ��������ǽ����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
���й�������Ħ������ļ���˵����ȷ���ǣ�������
| A��22.4 L�κ���������ʵ�����Ϊ1 mol | B����״���£�1 mol���ʵ����Ϊ22.4 L | C��H2��O2��N2��CO2��ɵĻ������1 mol�ڱ�״���µ����ԼΪ22.4 L | D����ͬ��ͬѹ�£���ͬ������κ����嵥��������������ԭ��������ͬ |
 ��1���ڱ�״���£�����A���ܶ�Ϊ1.25g/L������B������������ܶ�Ϊ21����8.96LA��B�Ļ�����������Ϊ13.44g����������A��B�������Ϊ
��1���ڱ�״���£�����A���ܶ�Ϊ1.25g/L������B������������ܶ�Ϊ21����8.96LA��B�Ļ�����������Ϊ13.44g����������A��B�������Ϊ