��Ŀ����
A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ��ԭ�ӵĵ��Ӳ���Ϊn������������Ϊ2n+1��A��B��C��Eÿ��Ԫ�ض�����DԪ��������ֻ��������ϵij���������ش��������⣺
��1��д�����и�Ԫ�ص����ƣ�A______ D______
��2����������Ԫ���У���һ��������С��Ԫ����______����̬ԭ��δ�ɶԵ���������ԭ�Ӻ�������Ų�ʽΪ______��
��3����֪��BA3DA��g��+A2D��g��=BD2��g��+3A2��g����H1=+49.0kJ/mol��BA3DA��g��+1/2D2��g��=BD2��g��+2A2��g����H2=-192.9kJ/mol����д������̬BA3DA��ȫȼ��������̬A2D���Ȼ�ѧ����ʽ�����Ƴ���Ԫ�ط�����д����______
��4���Կ��淴Ӧ3A2��g��+C2��g��?2CA3��g������Ӧ�ﵽƽ��λ��ʱ���ϸı����������ı�A2��C2��CA3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������CA3�ĺ�����ߵ�һ��ʱ����______����һ���¶��£�����4amol A2��2a mol C2����lL���ܱ������У���ַ�Ӧ����C2��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ______������ʱ�����������Ͷ��a mol A2��a mol C2��2a mol CA3���ж�ƽ���ƶ�����______�����������桱���ƶ�������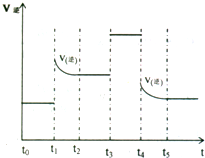
��1��д�����и�Ԫ�ص����ƣ�A______ D______
��2����������Ԫ���У���һ��������С��Ԫ����______����̬ԭ��δ�ɶԵ���������ԭ�Ӻ�������Ų�ʽΪ______��
��3����֪��BA3DA��g��+A2D��g��=BD2��g��+3A2��g����H1=+49.0kJ/mol��BA3DA��g��+1/2D2��g��=BD2��g��+2A2��g����H2=-192.9kJ/mol����д������̬BA3DA��ȫȼ��������̬A2D���Ȼ�ѧ����ʽ�����Ƴ���Ԫ�ط�����д����______
��4���Կ��淴Ӧ3A2��g��+C2��g��?2CA3��g������Ӧ�ﵽƽ��λ��ʱ���ϸı����������ı�A2��C2��CA3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������CA3�ĺ�����ߵ�һ��ʱ����______����һ���¶��£�����4amol A2��2a mol C2����lL���ܱ������У���ַ�Ӧ����C2��ת����Ϊ50%����÷�Ӧ��ƽ�ⳣ��Ϊ______������ʱ�����������Ͷ��a mol A2��a mol C2��2a mol CA3���ж�ƽ���ƶ�����______�����������桱���ƶ�������

Aԭ�Ӱ뾶��С����AΪHԪ�أ�BԪ��ԭ�ӵ��������������ڲ��������2������BΪCԪ�أ�CԪ��ԭ�ӵĵ��Ӳ���Ϊn������������Ϊ2n+1����nΪ2ʱ��CΪNԪ�أ���nΪ3ʱ��CΪClԪ�أ���������C����ΪCl������E�Ͳ��Ƕ�����Ԫ�أ�����CΪNԪ�أ�A��Eͬ���壬����EΪNaԪ�أ�A��B��C��Eÿ��Ԫ�ض�����DԪ��������ֻ��������ϵij����������DΪOԪ�أ�
��1���������ƶϿ�֪AΪHԪ�أ�DΪOԪ�أ���Ҫ��дԪ������Ϊ���� �����ʴ�Ϊ���� ����
��2�����ݵ�һ�����ܱ仯���ɣ����ڱ��У�ͬ���ڴ����ң�������������ͬ������ϵ��£���һ��������С������������Ԫ�����Ƶĵ�һ��������С��
H��C��N��O��Na������Ԫ�ص�δ�ɶԵ�������Ϊ��1��2��3��2��1��NԪ�ص�δ�ɶԵ�������࣬�����Ų�ʽΪ��1s22s22p3���ʴ�Ϊ��Na 1s22s22p3��
��3������ĸ���ɾ���Ԫ�صã���CH3OH��g��+H2O��g��=CO2��g��+3H2��g������H=+49.0kJ?mol-1
��CH3OH��g��+
O2��g��=CO2��g��+2H2��g������H=-192.9kJ?mol-1
���ݸ�˹���ɽ��ڡ�3-�١�2�ɵã�CH3OH��g��+
O2��g��=CO2��g��+2H2O��l��
��H=-192.9kJ/mol��3-49kJ/mol��2=-676.7kJ/mol��
�ʴ�Ϊ��CH3OH��g��+
O2��g��=CO2��g��+2H2O��l����H=-676.7kJ/mol��
��4����ͼ��ɿ������ı�����ƽ��һֱ���淴Ӧ�����ƶ�����t0-t1 NH3������ߣ�
3H2+N2=2NH3
��ʼŨ�ȣ�mol/L�� 4 a 2a 0
ת��Ũ�ȣ�mol/L�� 3a a 2a
ƽ��Ũ�ȣ�mol/L�� a a 2a
K=
=
������ʱ�����������Ͷ��a mol A2��a mol C2��2a mol CA3ʱ��
�൱�ڼ�ѹ��ƽ����������ٷ��������ƶ����ʴ�Ϊ��t0-t1
����
��1���������ƶϿ�֪AΪHԪ�أ�DΪOԪ�أ���Ҫ��дԪ������Ϊ���� �����ʴ�Ϊ���� ����
��2�����ݵ�һ�����ܱ仯���ɣ����ڱ��У�ͬ���ڴ����ң�������������ͬ������ϵ��£���һ��������С������������Ԫ�����Ƶĵ�һ��������С��
H��C��N��O��Na������Ԫ�ص�δ�ɶԵ�������Ϊ��1��2��3��2��1��NԪ�ص�δ�ɶԵ�������࣬�����Ų�ʽΪ��1s22s22p3���ʴ�Ϊ��Na 1s22s22p3��
��3������ĸ���ɾ���Ԫ�صã���CH3OH��g��+H2O��g��=CO2��g��+3H2��g������H=+49.0kJ?mol-1
��CH3OH��g��+
| 1 |
| 2 |
���ݸ�˹���ɽ��ڡ�3-�١�2�ɵã�CH3OH��g��+
| 3 |
| 2 |
��H=-192.9kJ/mol��3-49kJ/mol��2=-676.7kJ/mol��
�ʴ�Ϊ��CH3OH��g��+
| 3 |
| 2 |
��4����ͼ��ɿ������ı�����ƽ��һֱ���淴Ӧ�����ƶ�����t0-t1 NH3������ߣ�
3H2+N2=2NH3
��ʼŨ�ȣ�mol/L�� 4 a 2a 0
ת��Ũ�ȣ�mol/L�� 3a a 2a
ƽ��Ũ�ȣ�mol/L�� a a 2a
K=
| (2a)2 |
| a��a3 |
| 4 |
| a2 |
�൱�ڼ�ѹ��ƽ����������ٷ��������ƶ����ʴ�Ϊ��t0-t1
| 4 |
| a2 |

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ






 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������