��Ŀ����
ijʯ��Һ�����ɱ���Ͷ�����ɣ������������ֱ�Ϊ80%��20%������ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��C3H8(g)+5O2(g)====3CO2(g)+4H2O(l)����H=-2 200 kJ��mol-1C4H10(g)+![]() O2(g)====4CO2(g)+5H2O(l)����H=-2 900 kJ��mol-1��
O2(g)====4CO2(g)+5H2O(l)����H=-2 900 kJ��mol-1��
��һ����Ϊ0.80 kg���ݻ�Ϊ4.0 L����������һ��20 ���ˮ�տ�������Һ��ʯ����0.056 kg,�Լ����ȼ�ϵ������ʡ�
����֪ˮ�ı���Ϊ4.2 kJ��(kg����)-1�����ı���Ϊ0.88 kJ��(kg����)-1��
������1.0 kgʯ������ȫȼ���ͷŵ�����Ϊ��
![]()
��ˮ�տ���������Ϊ��
Q=cm(t-t0)=(4.2��4.0+0.88��0.80)��(100-20)=1 400(kJ)
���ԣ�ȼ�ϵ�������Ϊ��
![]()
�𰸣�50%

��ϰ��ϵ�д�
�����Ŀ
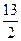 O2(g)==4CO2(g)+5H2O(1) ��H��-2 900 kJ��mol
O2(g)==4CO2(g)+5H2O(1) ��H��-2 900 kJ��mol